An Introduction to Industrial Hemp and Hemp Agronomy

Industrial hemp plants grown for fiber
Hemp became impractical as a crop in 1937 with the passage of the Marihuana Tax Act, which was an attempt by the U.S. federal government to reduce the production of Cannabis. The attempt to reduce production was largely due to the fact it was also being consumed as an intoxicant in addition to being utilized as a fiber crop. The new tax and licensing requirements made Cannabis production less profitable and much more bureaucratic, essentially ending production on large scales. There was a brief resurrection of hemp production during World War II, namely the Hemp for Victory campaign. During the campaign, U.S. farmers were asked to grow hemp for fiber to be used in the war effort. For instance in Kentucky, most hemp grown during the campaign was for seed to be shipped to northern states where it was planted as a fiber crop. All Cannabis became fully illegal to produce or even possess following the passage of the Controlled Substance Act (often abbreviated CSA) in 1978. Under the CSA, all forms of Cannabis were classified as Schedule 1 controlled substances and remain in that classification today. Other substances under Schedule 1 classification include the drugs heroin and cocaine.
The Agricultural Act of 2014, also known as the Farm Bill, provided for legal pilot research programs with industrial hemp to be administered by either state departments of agriculture or universities.
Cannabis sativa is a summer annual plant that is strongly photoperiod-sensitive (flowers according to day length/photoperiod; not physiological maturity). It is mostly dioecious in that male and female flowers occur on separate plants (i.e. there are both male plants and female plants). There are several monoecious commercial varieties (male and female flowers parts on the same plant). Different plant parts are harvested for specific purposes, and modern day hemp may be produced for one or sometimes dual purposes. Depending on the harvestable component of interest, (i.e. fiber, grain, or cannabinoids) male plants and/or pollen might be vitally necessary or completely unwanted.
Today, there is not much need for hemp rope or large hemp linens for sailing ships, etc., as there was in the nineteenth century; however, fibers from hemp possess several very positive attributes that may make them useful as modern natural fibers. Uses for hemp fibers today are different and much broader than when hemp was last grown across America. Fiber from hemp stems can be utilized in numerous ways ranging from low tech yarn and fabric to high tech electrical super-capacitors manufactured from biochar.
Other potential uses of hemp fibers could include alternatives for wood in construction materials (chip-board or particle-board), strength-increasing components added to concrete (hempcrete), or fiber used in composite materials in place of synthetic fibers (molded, injected plastics). A complete list of the potential uses for hemp fibers is too long to provide here.
The grain (seed) of hemp can also be used in numerous ways. As a dietary supplement for humans, it contains a very desirable ratio of omega-3 and omega-6 fatty acids compared to many other potential sources. It is also relatively high in both oil and protein contents. Hemp grain processors produce a wide array of consumer products including raw hemp hearts, toasted hemp seed, hemp seed oil, hemp flour, and even hemp coffee. It is also used as bird feed and livestock feed in Europe, either whole or in part. Again, an entire list of potential uses of hemp grain is very long. A common practice in regions where hemp is already being grown commercially is for producers to harvest hemp grain with combines and subsequently harvest the remaining stems for fiber similarly to harvesting a hay crop. This is the most common example of a dual-purpose industrial hemp crop. Soils climate are very conducive to hemp seed and grain production.
Cannabinoids are another harvestable component of Cannabis plants. They are plant-generated molecules that are known to have certain significant effects on humans. Cannabinoids are mainly, but not exclusively, produced by plants in the genus Cannabis. The most familiar cannabinoid is delta-9 tetrahydrocannabinol, or THC. This molecule is intoxicating and is responsible for the high obtained from using marijuana. By definition in the 2014 Farm Bill, the concentration of THC (by dry weight) defines a Cannabis plant as either marijuana (if it has more than 0.3% THC) or industrial hemp (if it is at or below 0.3% THC). This is the only distinction between marijuana and industrial hemp. They are the same plant species and look and smell exactly the same while growing. A simple analogy might be to compare sweet corn and field corn. They are both Zea mays L. and look very similar, but the biochemistry is very different between the two. The same is true for marijuana and industrial hemp.
There are dozens of other naturally occurring cannabinoids besides THC. One particular molecule, cannabidiol or CBD, is currently of deep and broad interest among pharmaceutical and medical researchers. Cannabidiol is reported to have strong potential for pharmaceutical applications. For example, certain epileptic patients treated with CBD experience reduction in the frequency and severity of seizures. There are other proposed, positive effects of CBD. Examples include potential uses as an analgesic, appetite enhancer, and anti-depressant. Many of the cannabinoids identified to date have not been studied for their potential as pharmaceutical agents. It is also thought that some number of cannabinoids remain to be identified.
Cannabinoids are present throughout the plant but are mostly concentrated in tissues from female flowers, especially in the leaf hairs of female flowers. Cannabinoids are found at much lower concentrations in root, shoot, and leaf tissues, and are not found in significant concentrations in hemp seed, seed oil, or pollen. In the case of optimizing cannabinoid production on a field scale, it is not known if the entire plant would be harvested and processed for cannabinoids, or just the female flowers. Field-scale cannabinoid production could be a case where male plants are totally unwanted. The concentrations of cannabinoids in male plants is very low compared to female flowers. Also, it is reported anecdotally that unfertilized (un-pollinated) female flowers tend to produce more cannabinoids than when they are pollinated and allowed to produce seed. As a result, in clonal propagation systems where all female plants are established, attempts would be made to prohibit male plants near the production field. Experiments are under way at UK to quantify the effects of pollination on cannabinoid production in both indoor and outdoor production systems. Early results support the anecdotal premise that un-pollinated female flowers produce more cannabinoids than pollinated flowers. However, this work must be repeated to provide a strong level of confidence in the results, and will be published as soon as that can be accomplished.
General Production Information
It is important to note that from the early twentieth century until 2015, there have been no U.S.-based agronomic research studies with industrial hemp. Information from historical research is important and useful but may not always be directly applicable to modern production systems. It is already very clear that different varieties of industrial hemp will respond very differently to our latitude and basic agronomic inputs. This is especially true regarding varieties grown for different purposes. Varieties grown for fiber-only will be established, managed, and harvested differently than varieties grown for grain or dual-purpose. Fiber and/or grain varieties will likely be established and managed very differently than those grown for cannabinoids. Until the information from these and other replicated research trials in the U.S. is available, we must rely on previous U.S.-based research and more recent research from other countries to make production recommendations (See Tables 1 and 2).
Basic Terms and Definitions
Pure live seed (PLS) is the seed in a container that will likely produce a viable plant when planted appropriately (depth, timing, etc.). Recommended seeding rates are always expressed as pounds of PLS/A. All containers of seed will have a mass or weight of materials in addition to PLS. This is usually expressed as a percentage of the total weight in the container. Examples include inert materials (soil, chaff, and other plant parts) as well as seed that will likely not germinate (immature or dead seed). Other contributions to reductions of PLS in a container are other crop seeds and weed seeds. The percentages of inert or other materials (non-crop seed) along with the germination percentage of the crop seed defines PLS. For example, a container includes 5 percent non-crop seed and has a germination rate of 75 percent. This means that 5 percent of the weight of the material in the container is not crop seed and that only 75 percent of the crop seed will likely germinate and produce a new plant. In order to calculate the amount of seed needed to accommodate recommended seeding rates, we need to increase the amount of seed planted by 30 percent above the recommended seeding rate (5%+25%; the sum of non-crop seed and crop seed that will likely not germinate). For example, if the recommended seeding rate is 60 pounds of PLS/A, we divide the seeding rate (60) by the PLS percentage (0.70 which equals 70%).
Table 1. General agronomic recommendations for the main harvestable components of industrial hemp
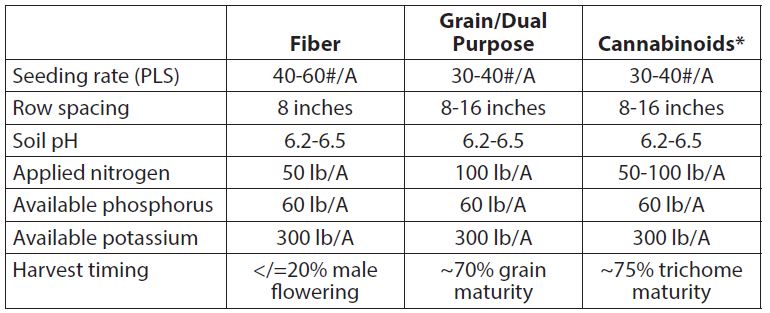
*Optimum agronomic protocols for cannabinoid production in field-scale systems have not been defined by replicated research methods. Much of what is practiced today is extrapolated from Cannabis production systems in U.S. states where it is legal and/or from other countries. Many production practices from these systems (e.g., fertility) pertain mostly to indoor and not field-scale production. Very important questions remain regarding field-scale systems to produce cannabinoids. These include understanding the effects of variety, establishment methods (e.g., direct seeding versus transplanting), and general crop management decisions including nitrogen fertility and harvesting/processing/storage issues. Research is under way to address these questions.
Table 2. Phosphate and potash recommendations (lb/A)
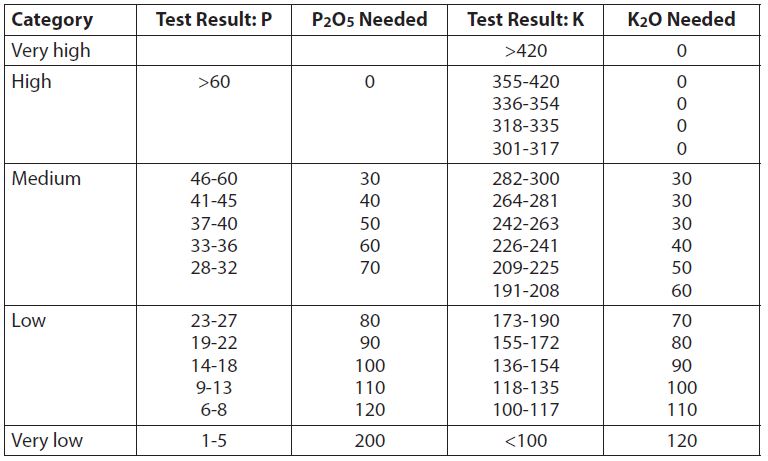
The result is we would need to apply approximately 86 pounds of this particular seed per acre to achieve the 60#PLS/A seeding rate. If germination percentages are not provided, it would be wise to have a germination test performed prior to seeding. This is true even if non-crop seed percentages are not available. We have witnessed very poor germination of some hemp varieties and have received several anecdotal reports of the same. Poor germination equates to poor stand establishment. Poor establishment leads to increased weed pressure, reduced yields, harvesting problems, or crop failures. Using the proper seeding rates based on PLS is imperative for successful hemp establishment and harvest. We note, too, that the 1,000-seed weights vary significantly among hemp varieties. We are working on recommendations based on the number of plants per square meter for successful establishment rather than pounds of seed per acre. The 1,000-seed weights can sometimes differ by two times among varieties, which would have a highly significant impact on plant populations and successful establishment.
Nutrient application rates are expressed as units or pounds of nutrient per acre. The amount to apply using a specific fertilizer is calculated by dividing the desired nutrient application rate by the analysis of the fertilizer. For example, to apply 50 pounds/units of nitrogen (N)/A using urea (46-0-0), we divide 50 by 0.46 (46% N in urea) which equals approximately 109 pounds of urea applied per acre.
Site Selection and Inputs
Although industrial hemp has been touted as a low-input crop that is highly adaptable to marginal lands, the scientific literature from other countries as well as research at UK clearly indicates that maximum yields are realized with inputs equivalent to current grain production systems (e.g., corn) and on productive land (>170 bu/A corn). If maximum industrial hemp yields are the goal, select good corn land (deep, well-drained soils) and plan on inputs equal to current grain crops. If maximum yields are not the goal, industrial hemp can be expected to perform on marginal lands with lower productivity and with reduced inputs much the same as our current commodity crops would.
Varietal Responses
Variety selection will be key to success for many reasons, most importantly days to maturity (latitudinal adaptation). There is much to know about selecting the proper variety. For example, varieties bred primarily for grain production could have significantly different maturity dates relative to each other, and therefore would have very different establishment dates for maximum yields and a crop that is harvestable with standard equipment. Producers should research varieties based on the harvestable component of interest (fiber, grain/fiber, or cannabinoids) and choose varieties that are proven performers. Standard industrial hemp variety trials are conducted at UK annually. The reports are posted on the UK agronomic hemp website at: http://hemp.ca.uky.edu/.
Establishment from Seed
It appears that industrial hemp seed is quite sensitive to a lack of soil moisture at planting. This trait has not been quantified but could readily contribute to stand failures. Seed should be planted in soils with adequate moisture to encourage rapid germination. If soil moisture is inadequate for industrial hemp germination, it is likely still adequate to support the germination of many weed seeds. Without the availability of labeled herbicides for industrial hemp production systems, we rely heavily on rapid hemp canopy development and closure to reduce or eliminate competition from weeds. Adequate soil temperature (>/=50oF) and moisture at planting will help accomplish this. Planting depth should never exceed one inch (1”), and 0.5 to 0.25 inch is preferred. Industrial hemp seed can be successfully drilled with both conventional tillage and no-till protocols. Seeding dates will depend equally on the harvestable component (fiber, grain, or cannabinoids) and the variety. Fiber crops will be harvested at the onset of reproductive growth and should be planted as early as possible to maximize vegetative growth (biomass production). Days to maturity of grain crops will vary a great deal among varieties. As such, some grain varieties should be planted much later than others to manage crop height at maturity. Optimal, field-scale cannabinoid production systems are not yet well-defined. Lacking appropriate research-based information, cannabinoid production from seed should be thought of similarly to grain production. In very general terms, industrial hemp seed can be planted in late April or early May. Seedling industrial hemp is tolerant of light frosts, but it is probably best to avoid the last killing frost while still taking advantage of good soil moisture and adequate soil temperatures common in spring.
Pesticides
There are currently no pesticides (herbicides, insecticides, fungicides, nematicides, etc.) labeled for use in industrial hemp crops in the U.S. This is true for both indoor and outdoor (field-scale) production systems. This means that any pesticide applications to industrial hemp crops are off-label and therefore illegal. Work is under way to evaluate pesticides for use in industrial hemp production systems and also to investigate several options for emergency exemptions within the rules and policies of the U.S. EPA. Today, it is imperative to make good management decisions to reduce the negative effects of pests, particularly weeds. Variety selection, soil moisture at planting, seeding dates, seeding rates, and fertility are examples of management decisions that will potentially reduce competition from weeds and increase yields without herbicides. To date, we have not witnessed significant pressure from insect or disease pests in field-scale production systems for fiber or grain. We are aware of reports of disease issues in field-scale cannabinoid productions systems, especially when clones are used as transplants.
Harvest Protocols
Harvesting industrial hemp grain by combine is the norm in other countries and has been accomplished successfully. Again, variety selection is key as the growth habits of those varieties bred primarily for grain production are more conducive to harvest by combine. Grain from varieties bred primarily for fiber production could be very difficult or perhaps impossible to harvest efficiently by combine, especially if planted early. Harvesting fiber crops is more complex. Fiber crops will nearly always require retting prior to baling. Retting is essentially a quasi-controlled rotting process. During retting, microbial activity breaks down the pectin layer between the bast and hurd fibers thus allowing for efficient separation. Microbial activity will be very sensitive to temperature and moisture. Generally speaking, warm and moist conditions will encourage microbial activity. The equipment for optimal cutting and then management of the crop during retting does not yet exist in the U.S. Additionally, field-retting industrial hemp will require new skills remotely similar to those involved in making high-quality hay. Successful field retting will be totally dependent on weather conditions just as is making good hay. Over-retting will dramatically reduce the quality of the fiber. Under-retting will make processing inefficient or impossible. Harvesting hemp stems for fiber with standard hay equipment can be difficult. Current thinking involves mowing by sickle-bar or mower-conditioner without conditioning/macerating, retting in the field, followed by baling (round or mid-size/large square). Optimal harvest and processing/storage methods for cannabinoids are not well defined in field-scale systems. Research is on-going at UK to address all of these questions.
D.W. Williams, Plant and Soil Sciences, and Rich Mundell, Kentucky Tobacco Research and Development Center