Extraction and Analysis of Tetrahydrocannabinol, A Cannabis Compound in Oral Fluid

Abstract
Although misuse and abuse of Cannabis is well known, the health benefits have been proved by various biomedical studies. Tetrahydrocannabinol (THC) is the major active substance in leaves of Cannabis, which is the common target for drug testing. In field drug testing, oral fluid (OF) has its unique advantages over other specimens such as blood, urine, and hair. Thus the study of THC in OF is gaining popularity in Cannabis research. In this review, extraction methods are introduced in three categories, which are Liquid-Liquid Extraction (LLE), Solid Phase Extraction (SPE), and Supercritical Fluid Extraction (SFE). Examples of application with each method will be covered. Advantages and disadvantages of these methods will be compared. In addition, methods in analysis following extraction will be briefly discussed.
1. Introduction
Cannabis is referred to the leaves, stems, flowers and seeds from one type of hemp plants, Cannabis sativa (National Institute on Drug Abuse, 2016). Cannabis is also known as marijuana and by many other names. In history, it has been considered as a highly potential drug for recreational use, misuse and abuse. It is classified as Schedule I drug in the United States (United States Code 21 U.S.C §802(32)(A); United States Code 21 U.S.C. §813). However, an increasing number of research had shown that Cannabis has plenty of health benefits for human, including treating glaucoma, controlling epileptic seizures and stopping cancer from spreading (NIHNational Eye institute, 2012; Pletcher, Vittinghoff, & Kalhan, 2012; Wallace et al., 2003). To date, more than 20 states in USA have legalized medical marijuana, as well as the recreational use in Colorado, Alaska, Washington, Oregon and District of Columbia (National Conference of State Legislatures, 2016). Michigan State is in the process of seeking legalization of the recreational use of marijuana (Michigan State, 2008). Thus, setting up the standard to extract and analyze Cannabis has become an urgent issue to ensure the purity and quality. The major active chemical in Cannabis is Δ9 tetrahydrocannabinol (THC), the primary metabolite of which is Δ(9)- tetrahydrocannabinol and 11-nor-9 carboxytetrahydrocannabinol (THC-COOH) (Goodwin et al., 2008). THC and THC-COOH can be extracted with various methods. Here we will summarize all the known methods in THC and THC-COOH extraction, as well as the related analytic techniques for them. Some law enforcement agencies in the world have employed the random roadside test of drug using oral fluid (Höld, de Boer, Zuidema, & Maes, 1996; Boorman & Owens, 2009). The roadside test often contains two steps. The first screening is done using a fast immunoassay, with limited accuracy and possibility of false positives. All the positive samples are subjected to a secondary confirmation by a formal laboratory test. The use of oral fluid (OF) has gained popularity over the recent years for its advantages over blood and urine samples, including its ease to collect, close supervision during collection and non-invasive nature (Gunnar, Ariniemi, & Lillsunde, 2005). In most of the studies, THC is the primary target analyte.

2. Review
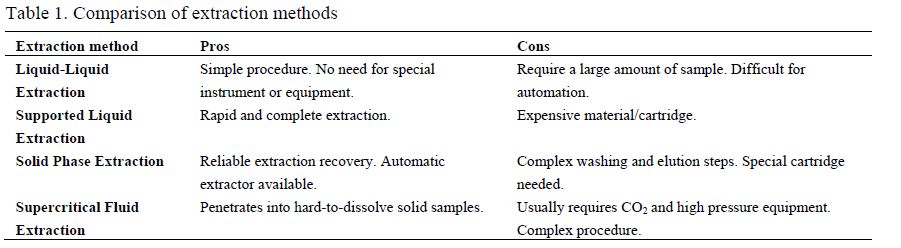
2.1 Liquid-Liquid Extraction
Liquid-liquid extraction (LLE) is a basic technique and widely used in analytical chemistry and often consists two types of immiscible solvents, usually aqueous solution and an organic solvent. The target analyte in most cases will be more soluble in the organic solvent. The analyte is usually in low concentration in the beginning solution. Using proper liquid-liquid extraction and drying will help to separate and concentrate the desired analyte and make it easier for the further analysis thereafter.
The liquid-liquid extraction method has been employed by many forensic and law enforcement agencies for the detection of drug abuse and driving under influence. For THC related compounds, most of them are more soluble in organic solvents than in water. So a simple direct organic extraction is adequate for the separation and enrichment of the analyte. An example can be seen in the study by B Backstrom et al. (1997). The herbal drug material was extracted using ethanol for 30 minutes at room temperature. Then the extract was analyzed against a standard solution. HPLC, GC-MS and SFC-APCI-MS were compared for the speed, selectivity, and reliability. In modern analytical testing environment, these techniques are commonly used as routine instrumentation. Basic HPLC and GC-MS flow chart and block diagrams can be seen in Figure 1-2.
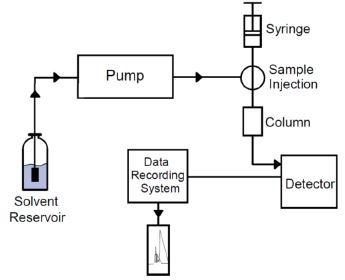
Figure 1. A Basic HPLC System Configuration
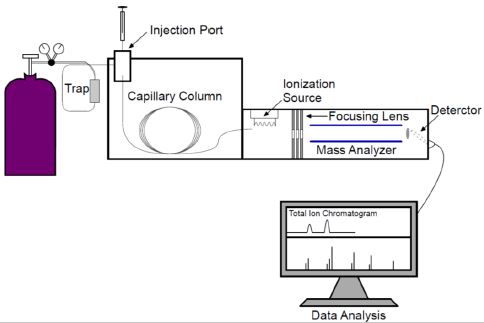
Figure 2. A Basic GC-MS System Configuration
Because of its simplicity and reliability, liquid-liquid extraction is used in several oral fluid analyses for the intoxication of THC. In the quantitation study of THC in oral fluid by Drummer et al. (2007), oral fluid (250 uL), buffer and 25ng of Δ3THC were added to extraction tubes with 1mL ammonium sulfate (1M, pH4.5). Then the mixture was extracted using 7mL of hexane. A freezing alcohol bath was used to freeze the aqueous phase. Thus the two phases can be easily separated. The organic part was then treated with pentafluoro-isopropanol and pentafluoropropionyl anhydride, and then was evaporated to dryness. The residual was then reconstituted with dry ethyl acetate and analyzed with GC-MS. The recovery of THC after the extraction was calculated to 60%-70%, with a reproducibility of 8.6% RSD.
Another new variation of liquid-liquid extraction is supported liquid extraction (SLE). This technique has gained popularity in human specimen analysis (Jiang, Cao, Zhang, & Fast, 2012). The principle of SLE is similar to regular LLE, which involves two immiscible solvents. In SLE, the aqueous phase containing the analyte is adsorbed onto a material with inert porous surface such as diatomaceous earth. The porous material will create a very large surface area of the liquid. When the second solvent is added, the mixing and extraction will occur on the large surface between the two solvents, thus the efficiency of extraction is increased. With the development of robotic sample preparation apparatus and 96-well plate sized cartridges, SLE becomes a more efficient and costeffective separation. Rositano et al. reported an application of SLE for the analysis of THC in oral fluid and blood samples in roadside drug testing (Rositano, Harpas, Kostakis, & Scott, 2016). For the oral fluid samples, 80 μL of 8% ammonia solution and 20 μL of internal standard was added to the 100μL of sample. Then the mixture was transferred into the Isolute® SLE+ plate. The sample was then eluted with 1mL of MTBE and dried with nitrogen at 35°C. The residual was reconstituted with 80 μL methanol. The subsequent LC-MS analysis successfully obtained a linear range of THC in sample of 0.9-56 ng/mL. The mean extraction recovery rate for THC in OF was 67%, with a relative standard deviation of 7%.

2.2 Solid Phase Extraction
Solid Phase Extraction (SPE) is widely used in drug analysis. A large variety of extraction cartridges are available commercially. The pre-filled syringe cartridges are often designed for rapid separation and preparation of samples. SPE is a technology derived from liquid chromatography. The stationary phase is often solid particle packing material. Different components in the sample solution can be separated chemically. SPE is commonly used for the selective removal of any possible interference in the sample matrix and simplify the preparation. Thus it will lead to a highly efficient and reproducible method. In drug screening analysis, THC is often analyzed simultaneously with many other drug substances such as Amphetamine, Cocaine, Morphine, and Codeine. In a validated method presented by Wood et al. a mixed-mode SPE was employed to ensure the complete extraction of all the drug substances, followed by LC-MS/MS analysis (Wood et al., 2005). This practice is common among the analysis of oral fluid, because the amount of oral fluid is usually very limited (Drummer, 2005). Introduction of SPE was investigated by Dams et al for the removal of the matrix effect. In Dams’ study, after SPE, the signal for morphine showed no significant suppression while small ion suppression was observed. Wood et al improved the SPE by using the Oasis® MCX (30mg, 1cm3) cartridge with mixed-mode and cation-exchange sorbents. This was carefully chosen to provide effective and selective separation for basic drug substances. There are three washing steps prior to the elution. First the cartridge with the sample was washed with 0.1N HCl solution to help retain the analyte and remove any salts in the solution. Then the second wash was performed with tetrahydrofuran to remove most of the surfactants. The third wash with methanol/water (50:50) to remove trace surfactants. After washing, the cartridge was dried and eluted with 0.5mL of 5% ammonia in methanol. This solution was treated differently for the unique properties of the analyte in interest.
Further investigation is needed for the full validated work of recovery rate and accuracy of THC extraction. And the LC-MS/MS analysis method is yet to be developed thereafter. An example of recent progression of the extraction and analysis of THC in oral fluid is the study by Badawi et al.
(2009). to simplify and automate the sample preparation and treatment, a Gilson SPE robot was used. The associated SPE column was Bond Elut Certify SPE (130mg, 3mL) from Varian. The SPE columns were conditioned with 2mL of methanol followed by 2mL of purified water. The oral fluid samples were diluted in a mixture of 0.1M ammonium acetate buffer at pH 4.1 and methanol (90/10, v/v). Then this solution was introduced into the SPE column at flow rate of 1mL/min. The two-step washing was carried out using first 2mL of purified water and second, 2mL of mixture of purified water and methanol (95/5, v/v). The eluting solution was a mixture of acetonitrile and 25% ammonium hydroxide (98/2, v/v). The loaded columns were eluted twice with 1.5mL of the eluting solution. The eluates were collected and evaporated at room temperature to dryness. Then it was reconstituted with 200uL of mobile phase that will be used in the following LC-MS/MS analysis.
To determine the recovery of extraction, two sets of samples, each contains 6 replicates, were evaluated. The samples in the first set were spiked with all analytes before the extraction while the second set of samples were spiked after the extraction. The recovery was calculated by comparing the absolute peak area of the analytes between the two sets. Recovery rates for most of the drugs are above 50% but only 33% for THC with a relative standard deviation of 19%. Nevertheless, the 33% recovery for THC is acceptable. When combined with the sophisticated LC-MS/MS method, the limit of quantitation was determined to be 0.5 ug/kg, the same as other 19 drug substances.
2.3 Supercritical Fluid Extraction
Supercritical fluid extraction (SFE) is the separation performed using supercritical fluid. Carbon dioxide (CO2) is usually used as the extracting solvent. When kept above 31°C and 74 bar, CO2 becomes as upercritical fluid. A phase diagram of CO2 is shown in Figure 2. Due to its very fast diffusion and very low viscosity, supercritical CO2 can penetrate into sample and dissolve the analytes in a short time, which is a major advantage for solid samples (Skoog, Holler, & Crouch, 2006). The drawback of SFE is the increased complexity of equipment and procedure.
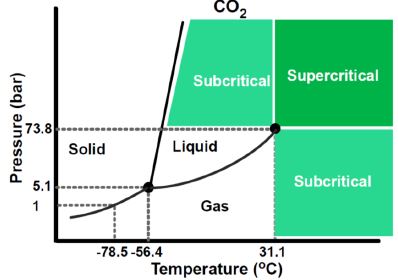
For this reason, SFE is only considered beneficial when other extraction and separation techniques do not produce a promising result. SFE has been employed in the analyzation of active substance in the Cannabis plant samples (Eöry, Dános, & Veress, 2001). The leaves are dried and grinded for uniformity. In this study, the powder was passed through sieves with different sizes, resulting of a series of particle sizes. All samples in the set were extracted with CO2 under supercritical conditions. The extractions were performed with 0.9 g/mL density of CO2 at 40°C. Then the analyte was eluted with 1.5mL of n-hexane. According to the extraction curve, the extractions were completed within 40 mins. The relationship between particle size (d) and THC content was discussed. Generally, smaller particles have higher THC content than larger particles. The peak content was seen at 1.6% in the sample with 0.063mm<d<0.125mm, while the coarsest sample with d>0.800mm contains only 0.5%, for comparison. There is a clear effect of grain size on the THC content of the sample. However, if all portions of the grinded sample were added together, the overall THC content as a whole is comparable to the total amount. This implies that the extraction was very complete regardless of particle size distribution.

3. Summary
Numerous extraction methods have been developed for the determination of THC in OF. The analyses of became popular in drug tests and forensic studies because its simplicity and security of sampling, especially in the field and/or on the road. The first step of testing is usually a fast-response screening. Any suspicious samples will be confirmed in an analytical laboratory. Thus, the fast confirmation that supports the legal decision is urgently required. The extraction and separation of the analytes from the matrix will greatly simplify and expedite the entire analytical procedure. In this paper, three major types including Liquid-Liquid Extraction, Solid Phase Extraction, and Supercritical Fluid Extraction are covered with detailed examples. The summarized comparison of the extraction methods is listed in Table 1 with advantages and disadvantages of each method.