Raphael Mechoulam

Cannabis – The Israeli Perspective
Abstract: Short overviews are presented on the historical uses of cannabis in the Middle East and on the more recent scientific and medical research on phytocannabinoids and the endocannabinoid system, with emphasis on research contributions from Israel. These are followed by examples of research projects and clinical trials with cannabinoids and by a short report on the regulation of medical marijuana in Israel, which at present is administered to over 22,000 patients.
Historical background
The resin of cannabis, hashish, has been used in the Middle East as a medicine, as well as a psychotomimetic, since ancient times. It is mentioned in Avesta, the Sacred Book of Knowledge of the Zoroastrian faith (1000–600 B.C.). It was well known to the Assyrians. Qunnabu is mentioned in Assyrian royal correspondence from around the end of 8th century B.C. apparently for use in traditional rites. Amongst its numerous uses as a medicinal agent, cannabis fumes were also prescribed as a treatment for the ‘poison of all limbs’ (presumably arthritis). In ancient Egypt, it was used as incense as well as medication for ‘mothers and children’. Today, we can only guess the nature of this ‘disease’. The Scythians, who ruled parts of present-day southern Russia, went south to plunder large areas of the Middle East (around 700 B.C.). Herodotus describes their use of cannabis as part of funerary customs. Burning cannabis and inhaling the smoke made them ‘howl in joy’.
Surprisingly, in ancient Judea, cannabis was apparently unknown. Or, if it was used as a medicine, it was ignored in the Bible, as it was part of the prevalent Assyrian customs and culture, which after the empire of Assyria disintegrated (around 6th century B.C.), the Judean leaders tried to suppress. However, it may be mentioned in the Bible as panag – an unidentified product exported to Tyre.
The Greeks and the Romans, who were in the Middle East for many centuries, were not aware of the psycho-activity of cannabis, but they used it as a medicinal agent, mostly for some types of pain and inflammations. However, Galen was aware that it produces ‘senseless talk’.
In medieval Arab society, for over a millennium, hashish was widely used for its psychoactive effects, although it was formally prohibited [3]. Its medical use is supposed to have been marginal, but the Jewish religious scholar, philosopher and physician Maimonides (12th century), who spent much of his life in Cairo, states that cannabis was amongst the most frequently used drugs [4]. And in a report, which obviously has modern-day implications, Ibn al-Badri tells that the epileptic son of the Chamberlain of the Caliphate Council in Bagdad was given hashish which cured him, but he had to continue smoking it throughout his life [3]. In the Middle East, since the Middle Ages and in modern times, hashish, although illicit, has continued to be widely used. Indeed, cannabis was introduced in Europe by Napoleonic soldier’s returning from Egypt.
Sporadic investigations on the chemistry, pharmacology and clinical effects of cannabis were reported throughout the 19th century. However, major advances had to wait until the 1930s when Cahn and Todd in the UK and Adams in the US initiated investigations in the chemistry and Loewe, initially in Germany and later in the US, in pharmacology. Cannabidiol (CBD) and cannabinol were isolated in pure form, and the structure of cannabinol was elucidated. Some synthetic molecules, with cannabinoid-like activity, were synthesized. However, the active principles were not isolated in pure form. Very little research was done in this field from the mid-1940s until the 1960s, mostly due to legal obstacles, which made work in academic laboratories extremely difficult.
Cannabis research – chemical and pre-clinical
The long tradition of hashish use in the Middle East and the absence of well-based chemical and pharmacological data in the field led my colleagues, Dr. Yuval Shvo and Dr.Yehiel Gaoni, and I, in the early 1960s, to reopen research in this area. We were able to obtain confiscated Lebanese hashish from the police. Using (then) modern methods of chromatography, we were able to isolate numerous cannabinoids and to elucidate their structures. These compounds were all tested for cannabinoid activity in monkeys by Dr. Habib Edery, and although all plant cannabinoids have closely related chemical structures, essentially only Δ9-tetrahydrocannabinol (THC) – originally named Δ1-tetrahydrocannabinol, which we first isolated in 1964 – no other compound caused significant sedation. At that time, this effect was the only one that we followed. Two additional compounds showed some activity: cannabinol, which is considered today to be an artifact formed on oxidation of THC, and Δ8–THC, which may be present in negligible amounts. Most of the cannabinoids isolated by us from hashish were synthesized by us and by other groups and became available for pharmacological and clinical research.
In the meantime, illicit use of marijuana had spread widely throughout the world, and a large number of investigators, particularly in the USA, initiated work on various aspects of cannabinoid effects. We learned a great deal on the metabolism, biochemistry and pharmacology of THC and to some extent also on CBD.
However, again due to legal constraints, very little clinical work was reported. Even today we have to depend mostly on anecdotal evidence or evidence based on small clinical trials as regards the possible clinical value of cannabinoids.
In spite of the advances in biochemistry, metabolism and pharmacology, the mechanism of action of THC, the major psychoactive constituent, remained elusive for nearly 20 years. It was originally believed that it acts nonspecifically on neural membranes. Gradually, pharmacological and chemical data surfaced, which were inconsistent with the non-specificity of THC action. Indeed, in the late 1980s and early 1990s two specific cannabinoid receptors, CB1 and CB2, were identified.
Stimulation of CB1 leads to the well-known marijuana effects; CB2 is apparently part of a major, new protective system.
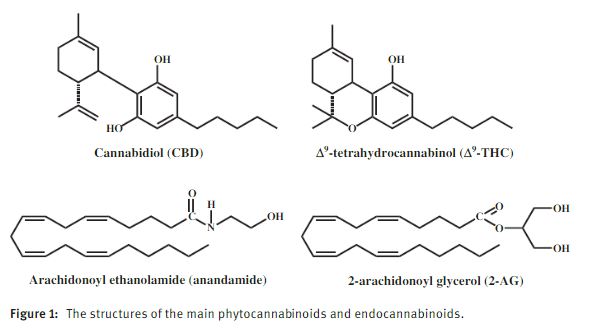
It is generally assumed that receptors are not present just to transmit effects caused by plant constituents. They are part of biological systems in which they are stimulated (or inhibited) mostly by endogenous molecules. In the early 1990s, we initiated a project to identify such endogenous cannabinoid compounds. We assumed that while such compounds will not be chemically related to THC, they would be lipid molecules as THC. We initially synthesized a tritium labeled THC-type derivative, which was bound to the CB1 receptor and then analyzed brain fractions for activity – namely release of the labeled compound by an endogenous molecule. After considerable efforts, we identified a novel compound, which was as potent as THC in binding to the receptor. We named it anandamide, based on the Sanscrit name for happiness and the amide part of the new molecule. Later, we discovered a second, chemically related molecule, 2- arachidonoyl glycerol (2-AG) in the periphery, and a Japanese group found it in brain [15]. Both anandamide and 2-AG are arachidonic acid derivatives. The structures of THC, CBD, anandamide and 2-AG are presented in Figure 1.
The cannabinoid receptors, the two endogenous cannabinoids (endocannabinoids) and the enzymes which synthesize and degrade them form the physiological endocannabinoid system (ECS), which is involved in a huge number of functions. Indeed, in a recent review [16], it was stated that ‘modulating ECS activity may have therapeutic potential in almost all diseases affecting humans, including obesity/metabolic syndrome, diabetes and diabetic complications, neurodegenerative, inflammatory, cardiovascular, liver, gastrointestinal, skin diseases, pain, psychiatric disorders, cachexia, cancer, chemotherapy-induced nausea and vomiting, among many others’ (with suitable references). This very strong statement indicates the importance of this new system.
The discovery of the ECS, coupled with the upsurge in cannabis use throughout the world, led to a major expansion of research in this field. Thousands of publications have appeared dealing with various aspects of the chemistry, biochemistry, pharmacology and, to a lesser extend again mainly due to legal barriers – the clinical aspects. For recent reviews, see references.
In Israel, the advances and local experience in chemistry and biochemistry and the availability of both phytochemicals and endocannabinoids led many groups to expand their research into the phytocannabinoid and endocannabinoid areas. Some of these projects were undertaken in collaboration with our Jerusalem group.
I shall try to summarize a few, very few, of the advances made by various groups in many of Israel’s universities and hospitals.
Brain trauma
A Hebrew University group in Jerusalem found that after closed head injury in mice, the level of the endogenous 2-AG was significantly elevated [20]. This effect was considered to be a protective reaction. Indeed, administration of synthetic 2-AG to mice after the injury was found to cause significant reduction of brain edema, better clinical recovery, reduced infarct volume and reduced hippocampal cell death. When 2-AG was administered together with additional inactive 2-acyl-glycerols that are normally present in the brain, functional recovery was significantly enhanced. This is another example of the ‘entourage effect’ noted previously, in which inactive brain constituents enhance the activity of endocannabinoids [21].Later, it was found that the brain constituents arachidonoyl serine and palmitoyl serine, which do not bind to the cannabinoid receptors, also reduce head injury [22, 23]. Unexpectedly, this effect can be blocked by cannabinoid receptor antagonists and is not noted in CB2 knock-out mice.
Bone effects
The group of the late Prof. Itai Bab at the Hebrew University initially found that both cannabinoid receptors are involved in the regulation of bone mass and bone remodeling, with the CB1 regulation taking place by modulating adrenergic signaling [24]. Later, it was found that the endogenous oleoyl serine (an anandamide-type molecule) also modulates bone mass and remodeling. In a recent publication which appeared after Bab’s death, it was shown that HU-433, a CB2 specific agonist, affects bone remodeling better than HU-308, another CB2 agonist, which, however, binds to the receptor with a much lower Ki, i.e. there is an inverse relationship between binding affinity and biological potency. This observation, if shown to be of general nature, may be of major importance in understanding of the nature of receptor binding. Another publication by Bab, which also came out after his death, reports that CBD enhances fracture healing – an observation of possible major clinical importance.For a review of Bab’s early work in the field of cannabinoids and bones, see reference.
Diabetes type 1
Another group in Jerusalem found that the administration of CBD to 11–14 weeks old female NOD mice, which were either in a latent diabetes stage or with initial symptoms of diabetes, ameliorated the manifestations of the disease. Diabetes was diagnosed in only 32% of the mice in the CBD-treated group, compared to 86% and 100% in the emulsifier-treated and untreated groups. In addition, the level of the pro inflammatory cytokine IL-12 produced by splenocytes was significantly reduced, whereas the level of the anti-inflammatory IL-10 was significantly elevated following CBD-treatment.
Arthritis
A collaborative effort of a Jerusalem group with a British one showed that in mice CBD ameliorated the clinical symptoms of arthritis induced by collagen [30]. CBD was equally effective when administered i.p. or orally. The dose dependency showed a bell-shaped curve, which is seen in many cannabinoid treatments. Clinical improvement was associated with protection of the joints against severe damage. Diminished IFN-γ production, as well as a decreased release of tumor necrosis factor by knee synovial cells, was noted. It was also found that CBD administration was capable of blocking the lipopolysaccharide-induced rise in serum tumor necrosis factor in C57/BL mice. Taken together, these data show that CBD, through its combined immunosuppressive and anti-inflammatory actions, has a potent anti-arthritic effect in collagen-induced arthritis.
Stress
A group at Haifa University has brought additional evidence on the existence of a relationship between glucocorticoids and the ECS. The ECS, which may be enhanced by stress, plays an important role in the downregulation of the hypothalamic-pituitary-adrenocortical (HPA) axis activity in response to stress; glucocorticoids affect the ECS to cause negative feedback control on the HPA axis during stress. Cannabinoid CB1 receptors are abundant in limbic regions where they may cause emotional arousal effects on memory. The Haifa group found that enhancing cannabinoids signaling, in part by glucocorticoids, prevents the effects of acute stress on certain kinds of memory.
Mechanisms of cannabinoid effects on the immune system
A group in the Weizmann Institute in Rehovot has shown that both THC and CBD potently reduce the Th17 phenotype, which is known to be increased in inflammatory autoimmune pathologies such as multiple sclerosis [32]. They found that reactivation of encephalitogenic T cells (cells that induce experimental autoimmune encephalitis when injected to mice), under certain experimental conditions led to a large increase in IL-17 production and secretion and that CBD and THC dose-dependently suppressed the production and secretion of both IL-17 and of IL-6, a key factor in Th17 induction. Pretreatment with CBD also resulted in increased levels of the anti- inflammatory cytokine IL-10. Surprisingly, these effects do not seem to involve the CB1, CB2, PPARγ, 5-HT1A or TRPV1 receptors.
Protective effects of ultralow doses of THC
A group in Tel Aviv University has found that a single ultralow dose of THC (0.002 mg/kg, several orders of magnitude lower than the conventional doses in mice) protects the brain from different insults, including inflammation, that cause cognitive deficits [33]. Mice received a single injection of low-dose THC; up to 7days after treatment with lipopolysaccharide (LPS), THC protected the mice from the long-lasting cognitive deficits caused by LPS. The protective effect of THC was blocked by a CB1 receptor antagonist but not by a CB2 receptor antagonist. The authors suggest that ‘an ultralow dose of THC that lacks any psychotropic activity protects the brain from neuro inflammation-induced cognitive damage and might be used as an effective drug for the treatment of neuro-inflammatory conditions, including neurodegenerative diseases’.
Clinical research
Epilepsy
On the basis of animal trials, our group, in collaboration with a Brazilian group [34], over 35 years ago, evaluated the effects of high doses of CBD in epilepsy patients. Fifteen patients received, in a double-blind procedure, 200–300mg daily of CBD or placebo for 4.5 months. Four of the eight patients that received CBD remained almost free of convulsive crises throughout the experiment, and three other patients demonstrated partial improvement. CBD was ineffective in one patient. In spite of these promising results, until recently no additional clinical work was reported. However, on the basis of a large number of anecdotal reports on the positive effects of CBD-rich marijuana on children with various pediatric epilepsies, several major trials have been initiated. Initial reports are positive. Why did we have to wait for decades?
Graft vs. Host disease
On the basis of the well-established effects of CBD on the immune system, a group at the Rabin Medical Center, associated with Tel Aviv University, initiated a clinical study on the possible treatment of graft vs. host disease (GVHD) – a reaction of the body to the replacement of bone marrow in certain types of cancer patients [35]. Forty-eight patients received 200–300mg/day CBD, and the results were compared to those of 101 historical control subjects given standard GVHD prophylaxis. The positive results were significant in both the patients that suffered from relatively mild and those with severe GVHD.
Post-traumatic stress disorder
In a small open trial, a group at the Hadassah hospital in Jerusalem showed that 5mg of THC, twice a day, as add-on treatment to the patient’s other drugs, led to significant improvement in sleep quality, frequency of nightmares and global symptom severity [36]. These results parallel related effects seen with a THC-like drug reported previously by a Canadian group [37]. They also give support to wide, illegal use of cannabis by post-traumatic stress dis-order (PTSD) patients.
Crohn’s disease
A group at the Meir Medical Center in Kfar Saba, associated with Tel Aviv University, has reported that in a placebo-controlled study, 21 chronic Crohn’s disease (CD) patients showed a significant decrease in a CD activity index. Ten out of 11 patients on cannabis improved clinically, compared to four of 10 on placebo [38]. While complete remission was noted in only one of 10 patients in the placebo group, five of 11 patients fully recovered in the group receiving cannabis.
Parkinson’s disease
A group at the Rabin Medical Center, associated with Tel Aviv University, assessed the clinical effect of cannabis on motor and non-motor symptoms of Parkinson’s disease (PD). Twenty-two patients with PD at a motor dis-order clinic were evaluated at baseline and 30min after smoking cannabis. Numerous scales were used to deter-mine the clinical effects. Significant improvement was noted in tremor, rigidity, bradykinesia and sleep and pain scores.
Legal use of medical cannabis
Around 10 years ago, the Israeli Ministry of Health decided to open the doors to treatment of various diseases with cannabis under strict supervision. A single physician who was head of a psychiatry hospital and continued his work as such – took upon himself to head this program. The administrative details changed over the years, but within 5–6 years, the procedure adopted was to allow only a specialist physician (not a general practitioner), treating a specific patient, to apply to the Ministry. The physician had to show that the conventional treatment that the patient had received previously was not efficient and that, on the basis of some published medical data, he/she believed that the cannabis treatment may be of value. Slowly, a list of ‘treatable’ medical conditions was compiled. It included chronic (mostly neuropathic) pain, some gastrointestinal conditions (including Crohn’s), pediatric convulsions and some neurological symptoms (trembling) but did not include psychiatric and most neurological diseases. However, a special committee could approve additional uses in particular cases, such as asthma. At present, there is a unit at the Ministry that is in charge of the program. About 22,000 patients have received medical cannabis, mostly for pain conditions but also for epilepsy (particularly in children), some gastrointestinal conditions, post-trauma, Tourette syndrome and others. The growers are required to supply medical cannabis in several forms – dry material for smoking or inhalation and oil solutions for oral administration. They also have to offer medical cannabis with different THC:CBD ratios: high THC and low CBD, high CBD and low THC and also THC and CBD in approximately equivalent amounts. The physician has to decide the type of medical cannabis required. There are numerous problems associated with the administration of medical cannabis:1. Not all medical conditions can be treated with the same ratio of cannabis constituents or with pure com-pounds. Thus, in GVHD and in epilepsy, pure CBD is apparently preferable than a mixture; in PTSD, possibly pure THC is a useful drug. But there are no hard data published.2. CBD is not available as a pure compound. Children with epilepsies are administered medical cannabis with a ratio of CBD:THC of about 20:1 or less, which means that a child administered 200mg CBD per day also gets 10mg (or more) THC. We know essentially nothing on the effects of chronically administered THC to a child whose central nervous system (which is obviously affected by THC) is in a stage of development.3. Physicians are used to administer drugs at well-defined levels, based on clinical trials. Most of them are not comfortable with the treatment of patients with a plant mixture, in most cases administered by smoking. Hence, many physicians refrain from get-ting involved in treatment with medical cannabis.
Concluding remarks
Israeli scientists have been involved in cannabis preclinical research over 50 years, and clinicians have done clinical research and have treated patients with cannabis for over a decade. But we still have to learn and get additional experience, particularly in the clinic. However, with the vast knowledge already available, it should be possible to advance rapidly. I see at least two possible directions:
1. CBD is a nontoxic molecule which does not seem to cause side effects. However, the doses needed are high. Thus, in schizophrenia, CBD has been assayed in the clinic with very positive results. But the doses needed are 800mg/day due to low bioavailability. Many natural products have been chemically modified to obtain better drugs. I assume that research on CBD will follow the same route and novel CBD-type compounds with a better pharmacokinetic profile will be developed.
2. Clinical trials are badly needed. They should be undertaken not only with the pure constituents but also in well-defined mixtures, previously evaluated in animal models and based on the possible ‘entourage effect.