Multiple sclerosis is a disease that affects the brain and spinal cord. Symptoms vary both in type and severity, but typically include pain, spasms, balance issues, tingling, vision problems and more. Research published in the Multiple Sclerosis Journal, see below, found that cannabis based medicinal extracts can significantly reduce the spasticity and pain associated with multiple sclerosis while having few adverse effects on patients.
Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients.
The objective was to determine whether a cannabis-based medicinal extract (CBME) benefits a range of symptoms due to multiple sclerosis (MS). A parallel group, double-blind, randomized, placebo-controlled study was undertaken in three centers, recruiting 160 outpatients with MS experiencing significant problems from at least one of the following: spasticity, spasms, bladder problems, tremor or pain. The interventions were oromucosal sprays of matched placebo, or whole plant CBME containing equal amounts of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) at a dose of 2.5-120 mg of each daily, in divided doses. The primary outcome measure was a Visual Analogue Scale (VAS) score for each patient's most troublesome symptom. Additional measures included VAS scores of other symptoms, and measures of disability, cognition, mood, sleep and fatigue. Following CBME the primary symptom score reduced from mean (SE) 74.36 (11.1) to 48.89 (22.0) following CBME and from 74.31 (12.5) to 54.79 (26.3) following placebo [ns]. Spasticity VAS scores were significantly reduced by CBME (Sativex) in comparison with placebo (P =0.001). There were no significant adverse effects on cognition or mood and intoxication was generally mild.
Randomized, controlled trial of cannabisbased medicine in central pain in multiple sclerosis
Abstract—Background: Central pain in multiple sclerosis (MS) is common and often refractory to treatment. Methods:
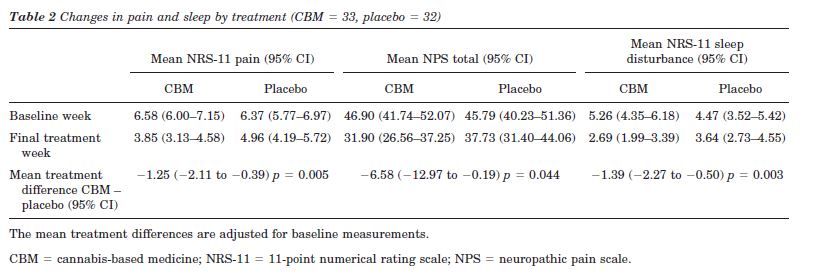
We conducted a single-center, 5-week (1-week run-in, 4-week treatment), randomized, double-blind, placebo-controlled, parallel-group trial in 66 patients with MS and central pain states (59 dysesthetic, seven painful spasms) of a whole-plant cannabis-based medicine (CBM), containing delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD) delivered via an oromucosal spray, as adjunctive analgesic treatment. Each spray delivered 2.7 mg of THC and 2.5 of CBD, and patients could gradually self-titrate to a maximum of 48 sprays in 24 hours. Results: Sixty-four patients (97%) completed the trial, 34 received CBM. In week 4, the mean number of daily sprays taken of CBM (n = 32) was 9.6 (range 2 to 25, SD = 6.0) and of placebo (n = 31) was 19.1 (range 1 to 47, SD = 12.9). Pain and sleep disturbance were recorded daily on an 11-point numerical rating scale. CBM was superior to placebo in reducing the mean intensity of pain (CBM mean change -2.7, 95% CI: -3.4 to =2.0, placebo –1.4 95% CI: -2.0 to -0.8, comparison between groups, p = 0.005) and sleep disturbance (CBM mean change –2.5, 95% CI: -3.4 to --1.7, placebo –0.8, 95% CI: -1.5 to -0.1, comparison between groups, p = 0.003). CBM was generally well tolerated, although more patients on CBM than placebo reported dizziness, dry mouth, and somnolence. Cognitive side effects were limited to long-term memory storage. Conclusions: Cannabis based medicine is effective in reducing pain and sleep disturbance in patients with multiple sclerosis related central neuropathic pain and is mostly well tolerated.
Central pain, i.e., pain initiated or caused by a primary lesion or dysfunction of the CNS, is estimated to occur in between 17% and 52% of people with multiple sclerosis (MS). As many as 32% of patients with MS regard pain among their most severe symptoms, confirming it as a “frequent, disabling and inadequately managed symptom.” The most common form of central pain in MS is nonparoxysmal extremity pain, which shows large inter individual variation and may manifest with several, typically dysesthetic, qualities such as burning, aching, pricking, stabbing, or squeezing. Painful extremity spasms have also been classed as central pain.
In the past decade, cannabinoids and the endocannabinoid system have come under intense scrutiny following the discovery of CB1 and CB2 receptors and development of specific cannabinoid receptor agonist and antagonist ligands. The rationale for performing a randomized, controlled trial (RCT) of cannabis-based medicine (CBM) in MS-related neuropathic pain is based on encouraging results using cannabinoid receptor agonists in relieving symptoms of experimental allergic encephalomyelitis7 and preliminary studies demonstrating modest positive effects of a synthetic cannabinoid analogue on neuropathic pain of mixed etiologies and of whole plant– derived CBM on neurogenic symptoms, including pain, in patients with MS. A systematic review of trials of cannabinoids in pain management concluded that cannabinoids may relieve neuropathic pain, but some authors have questioned the appropriateness of trials of cannabinoids using oral administration due to the variability in their gastrointestinal absorption and crossover designs because of their long half-lives.
This study was designed to evaluate the effect of oromucosal CBM in central pain associated with MS. CBM is derived from cannabis plant chemovars, developed to produce high and reproducible yields of specified cannabinoids and formulated to produce a CBM. Oromucosal administration is efficient and convenient in achieving accurate self-titration to overcome the wide variability of interindividual response known to occur with cannabis and cannabinoids. The study preparation contained a mixture of two principal ingredients of cannabis (delta-9- tetrahydrocannabinol [THC] and cannabidiol [CBD]) in approximately a 1:1 ratio, with small amounts (<10%) of other cannabis-based compounds, delivered via an oromucosal spray. We sought to compare the efficacy, safety, and tolerability of CBM THC: CBD with placebo in relieving central neuropathic pain in patients with MS.
Results
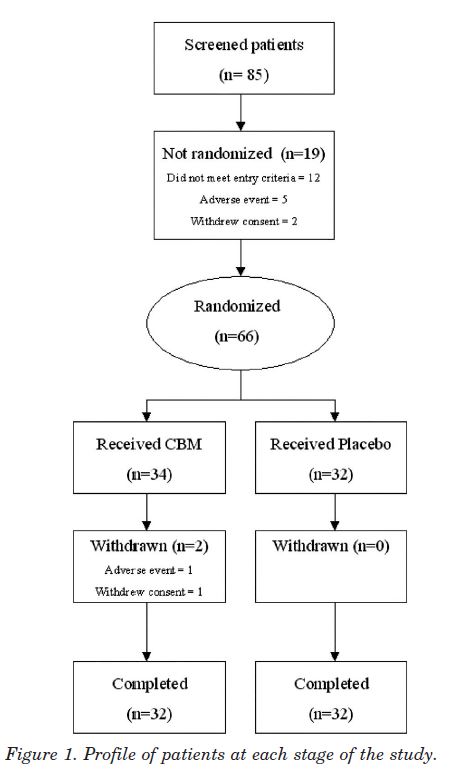
Study population. Recruitment from the regional MS clinic and referrals from consultant neurologists and pain specialists took place between March and July 2002, and patients attended the Trials Unit on four occasions over 5 weeks. Eighty-five patients were screened, of whom 66 were randomized (fig 1), 34 to CBM and 32 to placebo; 64 patients (96.9%) completed the study. Two women patients withdrew, both on CBM. One developed an AE of agitation with tachycardia and hypertension after four sprays, which settled with conservative management within 3 hours. She declined further study medication and withdrew 7 days later without completing further scores. The second patient developed paranoid ideation and was withdrawn from study medication at the investigator’s discretion in the second treatment week but subsequently completed all study diaries and assessments. Two patients violated the protocol, one patient’s concomitant pain medication changed in the run in period, and another patient commenced interferon treatment 3 days after commencing the study. Both patients were in the active treatment group and were included in the ITT analysis.
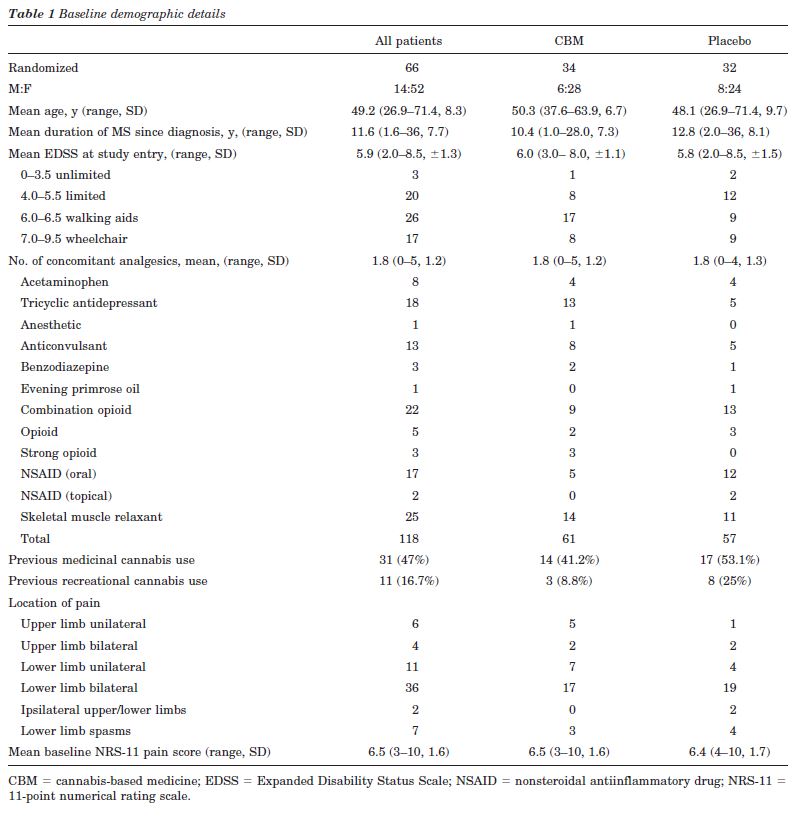
Of the 66 patients randomized, nine had primary progressive, 33 secondary progressive, 23 relapsing remitting and one benign MS. The treatment groups were well balanced in terms of gender, age, duration of MS since diagnosis, and baseline NRS-11 pain score (table 1). At baseline, the NPS items Intense, Unpleasant, and Deep were experienced by more than 80% of patients with mean severities of greater than four of ten. Conversely, less than 25% of patients experienced Cold and Itchy components with a similar magnitude and about 45% of patients did not experience these pain qualities at all.
Forty-three patients (65%) required support to walk or were wheelchair bound. Patients were taking a mean of about two other medications for pain, spasms, or spasticity. Forty-seven percent of patients had previous experience of using cannabis medicinally and 16.7% recreationally. Only four patients randomized to CBM and eight to placebo had taken cannabis within 3 months of study entry. The proportion of patients with any previous exposure to cannabis was not different between CBM and placebo (CBM 15/34, placebo 21/32; CBM-placebo -0.22, 95% CI:-0.45 to 0.02, p = 0.08). The mean number of daily sprays taken in week 4 was 9.6 of CBM (n= 32) (range 2 to 25, SD = 6.1), equivalent to 25.9 mg THC:24 mg CBD and 19.1 of placebo (n = 31) (range 1 to 47, SD = 12.9) (CBM-placebo -9.5, 95% CI: -14.6 to -4.4, p = 0.0004).
Efficacy measures
NRS-11 and NPS total pain scores. Significant mean reductions favoring CBM were found for the primary outcome NRS-11 of pain and the secondary outcome NPS (table 2 and figures 2 and 3). Of the total 65 patients included in the ITT analysis, 59 (89%) had dysesthetic pain and seven (11%) had painful spasms. Post hoc analysis demonstrated that the seven patients with painful spasms had higher baseline NRS-11 pain intensities than the patients with dysesthetic pain (dysesthetic pain 6.3 [SE =1.6]; painful spasms 7.3 [SE = 1.5]; difference in means - 1.3, 95% CI: 0.1 to 2.6, p = 0.04) and that the changes from baseline to week 4 tended to be greater in patients with painful spasms. In the patients with dysesthetic pain, the mean changes were -2.4 (SD = 1.5, n = 30) for CBM and -1.3 (SD = 1.7, n = 28) for placebo, whereas these changes were -5.7 (SD = 3.5, n = 3) and -2.1 (SD = 1.6, n = 4) in the patients with spasm. In an analysis including only the patients with dysesthetic pain, the NRS-11 pain treatment effect was 1.1 (SE = 0.4, p = 0.012). An interaction between treatment and type of pain was not found on a 5% level (p = 0.07).
Post hoc analysis of individual NPS items demonstrated treatment effects for all 10 items in favor of CBM that reach significance for Intense (treatment effect estimate –1.31, 95% CI: -2.21 to -0.40, p = 0.0054), Dull (treatment effect estimate –1.04, 95% CI: -2.05 to -0.03, p = 0.0433) and Sensitive (treatment effect estimate –1.01, 95% CI: -2.02 to -0.01, p = 0.0484).
Pain-related sleep disturbance. The reductions in pain were reflected in similar reductions in mean daily pain related sleep disturbance with a mean treatment difference favoring CBM (table 2, figure 4).
PGIC. The proportion of patients rating themselves as “much” or “very much improved” in the CBM group (9/ 34) was not greater than those receiving placebo (4/32) (treatment difference 14% points, 95% CI: -4.8 to 32.7, p = 0.218). No patient felt “much worse” or “very much worse” (see table E-1 on the Neurology Web site at www. neurology.org). On the 7-point PGIC, those treated with CBM were 3.9 times more likely to rate themselves in any improved category than those receiving placebo (95% CI: 1.51 to 10.09, p = 0.005).
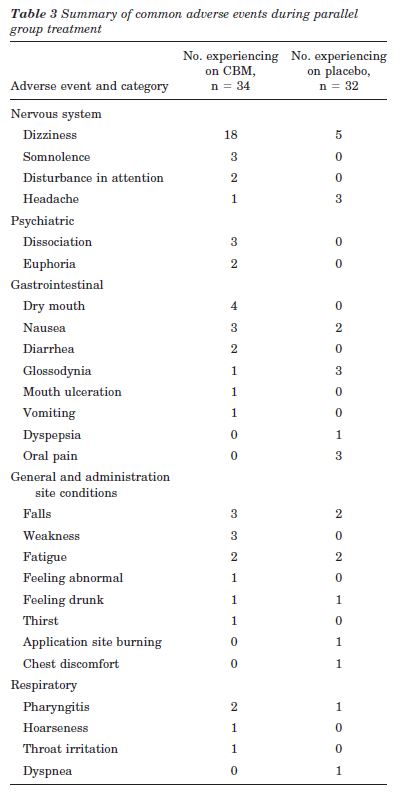
AEs. Thirty patients (88.2%) on CBM developed at least one AE, compared with 22 patients (68.8%) on placebo (CBM-placebo 0.19, 95% CI: 0.00 to 0.39, p = 0.053). Common AEs are summarized in table 3; in addition, confusion, crying, low mood, disorientation, paranoia, hallucination, and logorrhea all occurred once in the CBM group. Fifty-three percent of the patients in the CBM group experienced dizziness at least once compared to 16% in the placebo group (CBM-placebo 0.37, 95% CI: 0.16 to 0.58, p = 0.002). Some of the psychiatric AEs occurred in the same patient. No serious AEs, i.e., fatal, life-threatening, or resulting in persistent or major disability/incapacity or prolonging hospitalization, occurred. However, two women patients in the CBM arm experienced AEs severe enough to warrant trial withdrawal (see “Study Population” section).
No significant changes were seen in either group in blood pressure, weight, temperature, hematology, or blood chemistry. During the first dosing, there was an increase in mean heart rate in 1 hour in the cannabis-based medicine group by 3.2 beats per minute (95% CI: 2.3 to 4.1) compared with a mean of 1.6 beats per minute (95% CI: 0.8 to 2.5) in the placebo group, p = 0.016.
Neuropsychological outcomes. No significant differences between mean changes on each treatment were found in the 10/36 Spatial Recall Test, Symbol Digit Modalities Test, Paced Auditory Serial Addition Test, or Word Generation List between treatment groups on neuropsychological testing. In the long term component of the Selective Reminding Test (SRT), a difference was found because of a mean improvement in the placebo group (n = 32) of 5.7 (95% CI: -19 to 26) not matched in the CBM group (n = 33) of –0.9 (95% CI: -20 to 23) mean treatment difference -6.95 (95% CI: -12.12 to -1.77), p = 0.009 (see table E-2).
Other secondary outcomes. No differences between mean changes on each treatment were found between treatment groups in the other secondary measures of HADS anxiety and depression and Guy’s Neurological Disability Scale (see table E-3).
Discussion. This randomized, placebo-controlled trial demonstrates a beneficial effect of CBM both in the relief of central pain associated with MS and pain-related sleep disturbance. Although our study’s inclusion criteria allowed for any type of MS-related central pain of greater than 3 months’ duration, the predominance of dysesthetic extremity pain in this study (59 patients [89%]) is in agreement with previous series. The definition of and conditions encompassing “neuropathic” pain remain controversial. No universally accepted validated clinical diagnostic criteria for neuropathic pain exist, and assessment of patients based on clinical examination and bedside tests to decide what is and what is not neuropathic is difficult, even for experts.
Some authors view spasm-related pain in MS as being neuropathic, whereas others do not. A crossover trial of dronabinol in MS central pain specifically excluded patients with spasm-related pain. Painful spasms in MS feature sudden-onset, either unilateral or bilateral, dystonic posturing with a stereotyped pattern in the same patient. They are thought to be caused by “a transversely spreading ephaptic activation of axons within a partially demyelinated lesion.” No study to date has linked painful spasms to dysfunction in either the motor or somatosensory system exclusively, and whether there is any advantage in separating the two for therapeutic purposes is uncertain. Although patients with MS and painful spasm must have a CNS lesion, the key question is whether the pain is generated primarily in the spasmodic muscles or the CNS. In this study, patients with painful spasms responded similarly to those with predominantly dysesthetic pain, suggesting that dichotomizing patients based on putative differences in central mechanisms of the two groups may be superfluous.
Patients in our study were taking, on average, two other medications, with limited efficacy given baseline NRS-11 pain scores of 6.5. Therefore, as adjunctive analgesic treatment, CBM had a significant treatment effect of -1.25, in the NRS-11, in excess of the -0.6 achieved by oral dronabinol in an MS study in which concomitant analgesia was restricted to paracetamol and comparable to treatment effects of approximately 0.9 and 1.25 to 1.45 in RCTs of peripheral neuropathic pain using tramadol and pregabalin. The treatment difference for the NRS-11 did not reach that for which the study was powered, although this calculation was based on peripheral neuropathic pain studies. A meta-analysis of more than 2,700 patients with various painful conditions suggested approximately a 30% or 2-point NRS-11 score reduction in pain as being clinically significant28 but notably did not include patients with central neuropathic pain, in which “relatively small decreases in pain intensity are often highly valued by the patients.”
In our study, the numbers needed to treat29 to achieve a 50% reduction in central pain in at least one patient was 3.7 (95% CI: 2.2 to 13.0), similar to that obtained in the dronabinol trial of 3.5 (95% CI: 1.9 to 24.8). Numbers needed to harm29 (NNH) is calculated as 1/risk difference, and for the probabilities of at least one AE, this is 1/0.19 = 5.13. Specifically, for CBM to cause dizziness, the NNH was 1/0.37 = 2.68. Current options for treating central pain conditions remain limited and are based mostly on the use of CNS drugs with known problems of tolerability. CBM was well tolerated overall, despite a population including 25 of 34 patients (73.5%) in the treatment group requiring some walking aid and eight (24%) being wheelchair bound.
A systematic review identified that, until 2001, RCTs of cannabinoids were largely confined to single-dose trials. In this trial, patients could titrate to a maximum of THC 130 mg:CBD 120 mg. The mean dose achieved of 25.9 mg THC, and particularly 24 mg CBD, is in excess of that used in other cannabinoid RCTs.21,32,33 CBD is thought to modulate the effects of THC and also to have analgesic properties of its own. These factors may contribute to the positive outcomes in this trial. To place these reductions in patient’s pain in context, a quality-of-life instrument would have been beneficial, however, with a relatively short 3-week fixed treatment period. This was omitted from our study; however, an odds ratio of 3.9 favoring an improved global impression of change with CBM, without a corresponding significant change in mood, suggests that patients felt a benefit from reduction in pain, sleep improvement, or both and contrasts with a previous RCT using orally administered THC and whole-plant cannabis extract, which significantly reduced PGIC.
The Cannabinoids in Multiple Sclerosis (CAMS) trial using orally administered cannabinoid capsule formulations identified no objective change in Ashworth scores but did note subjective improvements in pain and sleep, which concurs with our results, as well as in spasms and spasticity.
In our study, the NPS 10-item total responsiveness also shows a significant treatment difference favoring CBM of -6.58 on a 100-point scale, demonstrating convergence with the traditionally accepted NRS-11 outcome. Post hoc analysis demonstrated significant treatment effects favoring CBM in the Intense, Dull, and Sensitive NPS items, suggesting that further studies should examine whether more sophisticated methods of analyzing the NPS are required.
Although unusual in neuropathic pain trials, some RCTs involving cannabinoids have included a question to formally assess the degree of blinding and demonstrated an element of unblinding in patients receiving cannabinoids.32,33 Our study did not include a blinding question. However, despite a number of our patients having previous exposure to cannabis, our placebo group experienced both a large reduction in pain and number of AEs, suggesting a degree of blinding was preserved.
CBM does not appear to have significant effects on MS-related disability, mood, or on four of the five neuropsychological outcomes measured. No corrections for multiple comparisons were applied to secondary outcomes. The significant treatment effect favoring placebo in the long-term storage component of the SRT, perhaps reflects a learning effect not matched in the CBM group. Analyses of the consistent long-term retrieval score and delayed recall at 11 minutes were not defined a priori for analysis in our study. The neuropsychological outcomes of chronic (10.2 to 24 years) recreational marijuana users and general population controls have been compared. On the Rey Auditory Verbal Learning Test, a significantly less steep learning curve and generally recall of fewer words were observed in long-term (mean 24 years) users of cannabis than in short-term (mean 10.2 years) users or controls. Long-term users also recalled fewer words than short-term users or controls. The preliminary results of the psychological sub-study of the CAMS trial found a significant reduction in the Californian Adult Verbal Learning Test in those receiving cannabis extracts compared with placebo.35 These results require further analysis and incorporation of psychological outcomes in future cannabinoid trials.