Targeting the endocannabinoid system may have a role in the treatment of post-traumatic stress disorder (PTSD). However, few studies have examined the effectiveness of cannabis on symptoms of PTSD, and more research is needed to ascertain cannabis’ effectiveness. In this retrospective naturalistic study, we followed 14 relatively mature (32-68 years of age), treatment-resistant, chronic combat post-traumatic patients who remained severely symptomatic despite treatment with many lines of conventional treatment prior to receiving medicinal cannabis. Our findings show that total sleep score, subjective sleep quality, and sleep duration significantly improved (p < 0.01). Total PTSD symptom score and its subdomains (intrusiveness, avoidance, and alertness) showed improvement (p < 0.05). However, there was no improvement in the frequency of nightmares (p = 0.27). The mean follow-up time was 1.1 ± 0.8 years (range of 0.5 to 3 years).
1. Introduction
Post-traumatic stress disorder (PTSD) is a mental disorder that can develop after a person is exposed to a traumatic event, such as threatened or actual death, serious injury, or sexual violence. Exposure can be direct, as a witness, as learning a traumatic event happened to a close person, or as repeated or extreme exposure to details of a traumatic event, such as during a line of work. The clinical presentation varies, and symptoms may include: Fear-based reexperiencing (such as in intrusive recollections, nightmares, or dissociative states); Intense physiological or psychological distress when exposed to triggering cues that remind of the traumatic events and persistent avoidance of such cues (internally or externally); Negative alterations in cognition or mood including difficulty in remembering important parts of the event, negative expectations about oneself, others or the future. Persistent negative mood states with decreased ability to feel positive feelings. Diminished interest in previously enjoyed activities and feelings of detachment or estrangement from others; Alterations in arousal and reactivity, including irritable behavior, hypervigilance, exaggerated startle response, and more (1). According to different guidelines for the treatment of post-traumatic stress disorder (PTSD), including the American Veterans Affairs (VA) and American Department of Defense (DoD) guidelines (2), and the International Society for Traumatic Stress Studies (ISTSS) (3), the first line treatment of PTSD includes psychotherapy and/or SSRIs or SNRIs. The most evidence-based and effective treatments are cognitive behavioral therapy (CBT), specifically prolonged exposure (PE), as well as eye-movement desensitization and reprocessing (EMDR) (4, 5). However, a large proportion of patients avoid psychological treatment, and the dropout rate among veterans is high (6). Remission rates with medication are only around 20 to 30%, and their side effects cause low compliance resulting in poor efficacy (7, 8). Considering the limitations of these treatments and the need for effective treatment for patients diagnosed with PTSD, an interest in medical cannabis for PTSD has risen in recent years.
In the past two decades, there has been growing literature implicating the involvement of the endocannabinoid system (eCS) in the etiology of PTSD (9, 10). The endocannabinoid system is a system of cannabinoids produced in our body and includes endogenous cannabinoids, such as N-arachidonoyl ethanolamine (anandamide) and 2-arachidonoyl glycerol (2-AG) and CB1 and CB2 receptors. CB1 receptors are located primarily in the brain and are widely located in the same areas in the brain that are involved in PTSD - the amygdala, hippocampus, and prefrontal cortex (11). CB2 receptors are located primarily in peripheral immunological tissue, although their presence in the central nervous system has also recently been documented. In fact, activating circuits and mechanisms involving CB receptors are similar to pathways involved in PTSD (12).
By activating CB1 receptors in the amygdala, cannabis can potentially reduce fear, anxiety, and aversive memories (13–18). By stimulating CB1 receptors in the prefrontal cortex, cannabis may increase serotonin levels, thus reducing depression, and improving mood, memory, and neurogenesis (17). Cannabis may also decrease hyperarousal and intrusive memories by activating CB1 receptors in the hippocampus and thus might help to reduce PTSD symptoms (13). Furthermore, studies demonstrate a lower concentration of endocannabinoids in patients with PTSD. For example, in a study by Hill et al., endocannabinoid levels were measured in 46 people (24 with PTSD and 22 without PTSD) who were exposed to the 9/11 terror attack. They found that 2-arachidonoylglycerol (2-AG) levels were significantly lower among those who developed PTSD (19).
Cannabinoids are a group of active compounds found in the cannabis plants. The most well-known cannabinoids are Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), which bind to the endocannabinoid receptors mentioned above. Both THC and CBD act on cannabinoid receptors CB1.CBD acts on CB2 too as well as other targets such as Fatty acid amide hydrolase (FAAH), serotonin 5-HT1A receptor and more (20). THC is the primary psychoactive ingredient in cannabis. It reduces anxiety (but can also increase anxiety), improves sleep, reduces nightmares, increases hunger, and helps in the extinction of fear memory (15, 21, 22). Cannabidiol (CBD) is a non-psychotomimetic cannabinoid that causes the least side effects while reducing the anxiety and psychoactive symptoms caused by THC (23). CBD significantly reduces the consolidation of aversive memories and has an anti-inflammatory effect with neuroprotective, analgesic, sedative, antiemetic, antispasmodic, anti-inflammatory, and anxiolytic properties (24). Cannabis use is not without its dangers. Cannabis use disorder afflicts about 22% of cannabis users, and 13% develop cannabis dependence (25). Cannabis use may increase the risk of psychosis and hinder its treatment (26, 27).
Literature reviews from the past two years that have examined the effectiveness of cannabis on PTSD symptoms count a small number of heterogeneous studies (open-label, longitudinal, and retrospective studies) with methodological problems and many limitations (28, 29). To date, there were only two randomized, controlled clinical trials for PTSD patients. The first used the synthetic cannabinoid nabilone (30), and the second used smoked cannabis but for only three weeks and was underpowered to detect significant differentiation from placebo (31). The conclusions of the few systematic reviews examining the effectiveness of cannabis on PTSD symptoms are that cannabis and synthetic cannabinoids may have a role in the treatment of PTSD, but there is currently limited evidence regarding their safety and efficacy (28, 29). Therefore, additional research is needed to better understand the effectiveness and safety of cannabis in the treatment of PTSD.
Israel has one of the longest-running medical cannabis programs in the world, starting in the 1990s (32). However, the use of medical cannabis for PTSD in Israel began only in 2014 (33). The use of cannabis for PTSD increased to almost 10% of total licenses by 2018, although Israel has a lower prevalence of PTSD than the USA (1.5% vs. 6.8%, respectively) (32). Medical cannabis is available as dried buds for inhalation or smoking and as an oil for sublingual ingestion. License for the medicinal use of cannabis for PTSD requires an application sent to the Medical Cannabis Unit at the Ministry of Health on behalf of a patient by the treating psychiatrist. Licenses are valid for a year and require that the psychiatrist request extending the permit annually (34). The initial dose is 20 grams per month, with possible increments of 10 grams at the treating physician’s request. We advised patients to start with a low-THC concentration strain. The cannabis is dispensed at specialized pharmacies given special permit by the Ministry of Health. According to the Ministry of Health guidelines at the time, medical cannabis may be prescribed to patients who are diagnosed with moderate PTSD and above, lasting at least three years, and characterized by great distress. Cannabis will only be given after at least two trials with different drugs and two psychological interventions have been attempted. Contraindications to treatment include a history of psychosis or drug abuse (33). In this study, we examined the effectiveness of medical cannabis among patients suffering from chronic combat-PTSD in a clinical setting in Israel.
2. Materials and methods
The study is a retrospective naturalistic study that used data meticulously gathered in a real-life clinical setting during routine follow-up unrelated to the study. It was approved by the institutional review boards and was conducted following the International Conference on Harmonization guidelines and ethical principles of the Declaration of Helsinki (approval# 0118-19-COM1). Patients consented verbally for their anonymized data to be examined with no patient declining.
Since 2015, our specialized psychiatric trauma unit has offered medical cannabis to treatment-resistant combat-PTSD veterans who fit the MOH criteria. Patients complete questionnaires every six months as part of their treatment follow-up and before applying for a medical cannabis license. The two questionnaires used are the Hebrew versions of the PSQI (35) and PDS (36).
The Pittsburgh Sleep Quality Index Hebrew version (PSQI-H) is a self-administered ten-question questionnaire (Cronbach’s alpha = 0.72). A previous study had the original English questionnaire translated, back-translated, refined, and later validated by fluent speakers of both English and Hebrew. We used the following questions: (A) subjective quality of sleep rated between 0 (very good) to 3 (very bad), (B) duration of sleep in hours, (C) Inability to fall asleep within 30 minutes rated between 0 (not in the past month) to 3 (three or more times a week), and (D) total score calculated from all ten questions (higher scores indicate worse sleep quality). The Posttraumatic Diagnostic Scale (PDS) is a 49-item measure that assesses all the DSM-IV criteria for PTSD and measures symptom severity. For this study, we only used part three of the questionnaire, which includes 17 questions that measure the severity/frequency of PTSD symptoms in the past month, as the diagnosis was already well established. Questions range between 0 (never or once in the past two weeks) to 3 (at least five times a week). Symptoms can be separated into reexperiencing, avoidance, and arousal clusters. Although a formal validation of the Hebrew translation of the PDS has not been published, several theses have implemented similar translations. Weisblum (37) reported that her translation had a satisfactory internal consistency level (Cronbach’s Alpha = 0.90) in a study of parents whose children underwent heart catheterization or cardiac surgery. We used Weisblum’s translation as we found it most accurate and faithful to the original English questionnaire.
We extracted data from the questionnaires given to patients diagnosed with combat-PTSD who started treatment with cannabis at the trauma unit at the Brüll Mental Health Center, Tel Aviv, between 2015 and 2018. The questionnaires were completed as part of the patients’ standard assessment just before receiving medical cannabis and every six months afterward.
This study included all patients who were treated with cannabis for combat-PTSD and who completed PDS and PSQI questionnaires both before starting treatment with cannabis and at least once afterward (no less than six months after starting cannabis treatment). In addition, these patients had to fit the criteria for medical cannabis treatment laid out by the Ministry of Health: (1) PTSD lasting at least three years (2) Moderate or severe severity of PTSD (3) At least two previous medications were used for at least two months, including SSRIs or SNRIs (4) Two psychotherapeutic treatments. Excluded for treatment by the MOH criteria: any history of psychosis or current psychosis or any substance abuse (specifically, patients in our sample stated they had no previous cannabis use).
Source: NHI GOV
Medical practitioners will be able to issue prescriptions for taking medical cannabis to an expanded list of patients with them needing a special license.
Access to medical cannabis will soon be considerably broadened in Israel to meet the needs of a growing number of patients, the Health Committee and the Israeli parliament decided Tuesday.
Thanks to the reform, which is expected to come into effect in the coming months, medical practitioners will be able to issue prescriptions for taking medical cannabis to an expanded list of patients without them needing a special license.
Epilepsy, Crohn's disease, dementia, autism, oncological diseases, multiple sclerosis, HIV or AIDS, and terminally ill patients whose life expectancy does not exceed six months: All patients with these diseases will see their access to medical cannabis greatly facilitated with a simple prescription from a doctor in the same way as other drugs. At the same time, medical professionals will be trained on the issue of kashrut – religious dietary requirements – affecting medical cannabis.
Currently, patients who want to use medical cannabis must go through complex procedures that lengthen the waiting period. They thus need to obtain a special license from the Health Ministry, which is granted only by certain doctors trained for this purpose.
Some 100,000 patients have such licenses in Israel to consume medical cannabis, including many who suffer from post-traumatic stress. These patients voiced frustration that the syndrome they suffer from is not included in the list of diseases that are accepted for access to medical cannabis.
The chairman of the Health Committee, however, stressed that this was only a first step and that the list could be expanded.
Aharon Shavi, director of the drug treatment service of the Welfare and Social Security Ministry, has called for increased vigilance so that this expansion of prescriptions is not used to fuel addictions or illicit cannabis trafficking.
Source: i24 News Israel

Dr. Bruce Bugbee president and CEO of Apogee Instruments, Inc. A professor and researcher at Utah State University. R&D and production of Apogee has grown into a world-class manufacturer of environmental sensors, a state-of-the-art headquarters and manufacturing facility. The keys to Apogee's success has been Dr. Bugbee passion for creating top-quality, cost-effective instrumentation for scientists that meet his own rigorous performance standards in the field and the lab.
Video: Maximizing Cannabis Yields for Home Growers with Dr Bruce Bugbee
First discovered in Israel in the 1960s by Professor Raphael Mechoulam and his team, cannabinoids are naturally occurring chemical compounds derived from the cannabis plant. These compounds are responsible for the many medicinal effects of cannabis, with each compound offering distinctive properties and benefits. To date, scientists have discovered over 113 cannabinoids, and more are likely to be found.
Cannabinoids
Your body responds to every cannabinoid compound differently thanks to a remarkable built-in mechanism: the endocannabinoid system. This complex system is made up of receptors scattered throughout the body, which regulate health and homeostasis. The receptors have been identified in nearly every major organ system, from the brain and spinal cord to the gastrointestinal tract. CB1 receptors are associated most closely with the brain and nervous system, while CB2 receptors are linked to the immune system. These receptors, along with enzymes that aid in cleanup after many endocannabinoid system processes, help our bodies maintain a stable internal environment.
When activated by exposure to cannabinoids, the receptors of the endocannabinoid system become reactive. This means they’re able to affect key body processes including mood, memory, appetite, and pain. The specific effects of cannabis-derived products depend on the particular compound used and the location of the receptors that bind with that compound; we’ll look more closely at the receptor-cannabinoid interactions of various CBD compounds in the sections below.
CBD
The most familiar of the cannabinoids is CBD, an abbreviation that’s short for cannabidiol. Unlike THC, the other well-known compound derived from cannabis, CBD doesn’t have psychoactive effects. That means you can use it for medicinal purposes without getting high, so it’s safe to utilize even when you’re planning to work or drive. CBD is also an extremely adaptable compound, so it can be transformed into oils, gummies, pills, creams and more to suit various therapeutic needs.
CBD is the best researched of the cannabinoid compounds, and its applications are exceptionally wide-ranging. Studies show that CBD can be used for:
CBD — Reported Therapeutic Effects
1. Anxiety and stress
2. Depression
3. Schizophrenia
4. Panic
5. Withdrawal symptoms in cannabis and tobacco addiction. Inhibition of the reward-facilitating effect of morphine and cocaine.
6. Auto-immune diseases (diabetes type 1 for example)
7. Auto-immune-like diseases (GVHD, for example)
8. Conditioning
9. Inflammation (Crohn's disease, colitis, pancreatitis, rheumatoid arthritis).
10. Reduces infarct size and increase blood flow in stroke;
11. Obesity (food consumption; lowering appetite); metabolic syndrome.
12. Retinopathy associated with diabetes.
13. Antiemetic and anti-nausea
14. Protects against myocardial, liver, renal ischemic/reperfusion injury
15. Protects against hypoxia/ischemia injury.
16. Neuroprotection against neuronal damage due to neurological diseases or injury (Parkinson's disease; Huntington's disease; Alzheimer's disease; cerebral infarction; hepatic encephalopathy; traumatic brain injury; cerebral ischemia; spinal cord injury; memory rescuing effects; ).
17. Cancer and resistance to cancer chemotherapy; cancer cell migration (metastasis); inhibits angiogenesis.
18. Epilepsy and convulsions.
19. Chronic inflammatory and neuropathic pain
20. Lowers cannabis and THC effects such as memory loss, psychotic-like symptoms, anxiogenic action
21. Protects against airway obstruction
22. Obsessive-compulsive behavior
23. Memory rescuing effects due to neurodegenerative disorders
24. Epigenetics
25. Reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia
26. Restless leg syndrome
27. Disrupts the consolidation of specific and generalized fear memories
28. Preventing the development of chemotherapy-induced peripheral neuropathy
29. Osteoarthritis
30. Gout
31. Kidney injury
32. Familial Mediterranean fever (auto-immune)
33. Aggressiveness
34. Cannabidiol Improves Cognitive Impairment and Reverses Cortical Transcriptional Changes Induced by Ketamine, in Schizophrenia-Like Model in Rats
CBDA
Cannabidiolic acid, generally abbreviated to CBDA, is a cannabinoid produced by the stems, leaves, and flowers of some cannabis plants. Through a process called decarboxylation, the acid is removed from CBDA, transforming it into CBD. This process is most often performed by heating or smoking cannabis varieties that are high in CBDA. For this reason, CBDA is sometimes considered the “precursor” to CBD.
CBD and CBDA share similar molecular structures, so their therapeutic effects are also similar; however, CBDA has been the subject of less extensive scientific study. Scientists have learned that CBDA works primarily as an inhibitor of the COX-2 enzyme within the endocannabinoid system, leading to exploration of its effectiveness as a treatment for inflammation. Recent studies have also tested the efficacy of CBDA for certain types of cancer, and as an anti-emetic.
CBN
CBN is the abbreviation for cannabinol, another compound within the cannabinoid family. In fact, CBN was the first cannabinoid isolated by scientists. CBN is produced when THC is heated or exposed to oxygen; it also occurs naturally as the cannabis plant ages. Even though CBN is derived from THC, it doesn’t share the psychoactive properties of THC.
Within the endocannabinoid system, CBN binds to receptors less effectively than many other cannabinoids. However, it has been studied extensively as a helpful compound to improve sleep health. Scientists have discovered that CBN acts as a powerful sedative, with effects comparable to common sleep-inducing pharmaceuticals like diazepam. In studies on mice, CBN has been shown to prolong sleep time; additional studies suggest that this effect is amplified when used in combination with THC.
Along with its implications for sleep health, CBN has been studied as a possible stimulant for bone tissue growth. Research shows that it may activate stem cells that facilitate the production of new bone, making it potentially useful for the healing of fractures. Additional studies have explored the analgesic, antibiotic, anticonvulsant, and anti-inflammatory applications of CBN.
CBG
Like the other compounds in this overview, CBG (short for cannabigerol) is a non-psychoactive cannabinoid with a variety of promising medical applications. CBG is actually the precursor to its more famous cousins, CBD and THC. Like CBDA, exposure to light or heat breaks down CBG in the cannabis plant into these better-known compounds.
Most strains of cannabis contain relatively little CBG, often less than 1%. However, that doesn’t mean this cannabinoid is any less promising when it comes to potential applications. CBG interacts with both CB1 and CB2 receptors in the endocannabinoid system; during these interactions, it’s thought to naturally increase dopamine levels, which help to regulate sleep, mood, and appetite. CBG is also thought to obstruct GABA uptake in the brain and block serotonin receptors—both positive implications for the treatment of anxiety and depression.
Studies have found CBG especially effective for certain physiological systems and symptoms, including:
Glaucoma
Endocannabinoid receptors are highly concentrated in the structures of the eye, and CBG has been shown particularly effective at reducing the intraocular pressure associated with glaucoma.
Cancer
A recent study offered promising results for CBG as a cancer-fighting compound, with the potential to block the receptors that cause cancer cell growth. Scientists saw inhibition in the growth of colorectal cancer cells in mice that were treated with CBG, offering an exciting new avenue of treatment for cancer patients.
CBC
Discovered more than five decades ago, cannabichromene (abbreviated CBC) is considered one of the most promising cannabinoids in recent medical research. Like CBD and THC, CBC is derived from CBDA when the acid is broken down by exposure to heat or ultraviolet light.
Non-intoxicating like other CBD compounds, CBC is less well researched than some cannabis derivatives. However, scientists have discovered a variety of potential applications for this cannabinoid.
Within the endocannabinoid system, CBC binds most effectively with vanilloid receptor 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1); both of these receptor types are linked to the body’s perception of pain. This means that CBC may function as an alternative to traditional painkillers like NSAIDS, but without their potentially harmful side effects. CBC may be particularly effective for treating inflammatory conditions like osteoarthritis, especially when used in combination with THC.
Additional studies have shown that CBC may be a potential cancer fighter, second only to CBG in inhibiting the growth of cancer cells. Though research in this field is limited so far, the anti-inflammatory properties of CBC may also make it an effective acne treatment; studies suggest that it could work to prevent the sebaceous gland inflammation at the root of many types of acne.
While these therapeutic benefits overlap with many other cannabinoids, CBC is differentiated by what’s known as the “entourage effect.” Researchers believe that CBC may work synergistically when used with other cannabinoids to provide even more effective treatments for many of the conditions outlined above.
CBDV
Last in our roundup of cannabinoid compounds is cannabidivarin, better known as CBDV. CBDV is extremely similar to CBD on a molecular level, but recent research has shown its applications are exceptionally unique and valuable for people with neurological disorders.
Preliminary studies on mice show that CBDV has enormous untapped potential in the treatment of epilepsy and similar neurological conditions. As an anticonvulsant and antiepileptic, CBDV may be able to help patients who suffer from epilepsy, Parkinson’s disease, and other conditions in which seizures may occur. Along with reducing the duration and intensity of seizures, CBDV could work to prevent convulsions in the event that a seizure does occur. Early research on these applications for CBDV is so promising that GW Pharmaceuticals, a cannabis-focused company based in England, is working to patent the use of CBDV for the treatment of seizures.
Along with seizure treatment, CBDV may be used by patients who experience vomiting and nausea, especially when those conditions are caused by chemotherapy. It has also been studied as an appetite suppressant, and as a treatment that relieves symptoms of Crohn’s disease and multiple sclerosis.
Like all of the cannabinoids discussed above, CBDV is non-psychoactive.
Disclaimer: These statements have not been evaluated by the Food and Drug Administration. Products discussed are not intended to diagnose, treat, cure, or prevent any disease.
The new Israeli cannabis company "Univo" reports that it has basically agreed to import about half a ton of medical cannabis from the Canadian cannabis company "Canopy".
470 kg of cannabis will be imported into Israel from the world's largest cannabis company, says the new Israeli cannabis company UNV Medicine LTD. Univo which will be the exclusive importer of cannabis in Israel from Canopy.
Cannabis's products that will be shipped to Israel were not designed to be labelled Canada's famous brand, including "Tweed" and "Spectrum", but would be branded as "Unvio" products.

The announcement of the import from "Canopy" comes after the import of 250 kg made by Canndoc Ltd. "Canndoc" from the Canadian cannabis company "Tilray", and 400 kg of cannabis imported by the "Bazelet" members of the Portuguese farm company "Emek". In addition, the company "Together" announced it will import 250 kg of cannabis from its cannabis farm in Uganda.
After Tweed Canopy's imports are realized in Israel, cannabis from overseas of a total weight of over 1.3 tons, following the Ministry of Health approval for companies to import in view of the status of local cannabis deficiency.
This phenomenon of importing cannabis from abroad is expected to seriously harm small Israeli farmers who hoped to establish Israeli cannabis reviews for marketing the produce to factories. Collaboration agreements with the largest Canadian companies in the peripheral world, a large part of the activity share in this area.
According to Univo's announcement to the stock exchange: "Along with exclusive engagement in Israel, Univo will first import 470 kilograms of dry medical cannabis grown by canopy, produce its products with the cannabis raw materials that carry Univo's logo and brand and find the national cannabis products. Exclusively, as final packaging in pharmacies for the Israeli market. ”
It is also reported that "Univo's imported product is the GACP librarian, from which Unibo will produce GMP paper products and subject to the certification and instructions of the Medical Cannabis Unit of the Ministry of Health. The second home of the European Cannabis Medical Memorandum and Memorandum in Europe, the European market For the required permits. "
Golan Bitton, Univo's CEO, said: "The agreement with Canopy is a move. In addition, the agreement is proof that there is a right in establishing a technology enterprise with a larger capacity that the technology will produce products of international quality and standards. The agreement will also allow us to define our market share in Israel, regardless of our farms and professional supplies and continue to sell to expand our range of medical cannabis products.
Source: Cannabis Magazine

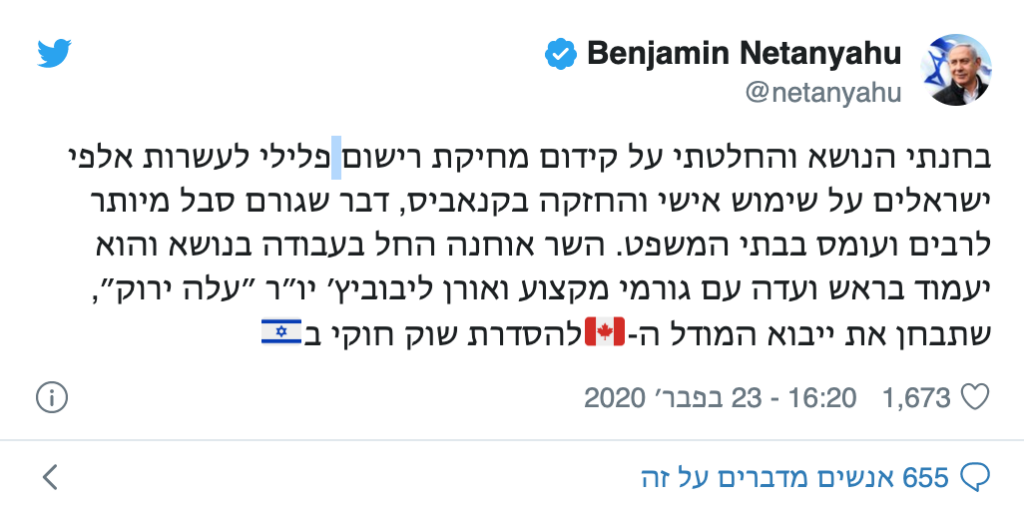
On the way to legalization in Israel: After a round of discussions with the leaders of the struggle, the PM agreed on a full pardon for cannabis consumers (deletion of criminal cases).
After a round of discussions during which meetings were held between Prime Minister Benjamin Netanyahu and Chairman of the Green Leaf Movement and editor of Cannabis Oren Leibowitz magazine and between Justice Minister Amir Ohana and Attorney Hatz-David Ozer, the PM decided to adopt the main elements of the "Leaf Plan" green.
The plan includes retroactive amnesty and deletion of criminal records of self-use of cannabis for some 40,000 Israelis, and the establishment of a committee to promote the legalization of a legal cannabis market in Israel (legalization), including opening legal stores and permitting domestic growth.
During the meetings, leaders of the legalization movement in Israel presented Leibowitz and helped Netanyahu and her data on the current harms of the anti-cannabis consumer policy and details on Canada's successful legalization model.
The petition details presented by the two on behalf of thousands of supporters (the High Court of Cannabis), including a demand for a complete abolition of the market and regulate the market in a controlled manner (legalization). The first hearing in the future will take place in the High Court in two months (22.4).
Apart from eliminating criminal cases in the context of mass pardon and the establishment of the first legalization committee in Israel, the parties intend to promote immediate solutions to reduce medical cannabis prices in pharmacies - these have jumped to an all-time high as a result of industry reform.
Benjamin Netanyahu
@netanyahu
I examined the issue and decided to promote the deletion of criminal record for tens of thousands of Israelis for personal use and possession of cannabis, causing unnecessary suffering to many and burdens in the courts. Minister Ohana began work on the issue and he will chair a committee with professionals and Oren Leibowitz, chairman of Green Leaf, who will examine the import of the model in Canada to regulate a legal market in Israel
A first announcement of the program was posted on Twitter on Twitter and Facebook symbolically at exactly 4:20 this afternoon. More updates are expected later this week. The legalization committee will be formed soon and will be chaired by Minister Ohana Belibowitz.
This decision is a continuation of what Netanyahu said in the April 2019 elections, when he stated that he intended to abolish the inclusion of cannabis consumers. Ahead of the second round of elections in September 2019, Netanyahu has already announced steps to legalize. In the meantime, he said he would support a bill that would allow cannabis plant growth in the home.
Justice Minister Ohana also commented on the issue when he expressed support for full legalization several times. The current decision is another step forward, this time more practical, to the realization of the fight for legalization, should the right bloc reach 61 seats.
Source: Israel Cannabis Magazine
According to the old regulation, the price of medical cannabis in Israel stood at NIS 370 a month - regardless of the amount of cannabis licensed. So how much does medical cannabis cost and how much money is spent on each month?
In 2009, the medical cannabis industry was first established in Israel. Initially, cannabis raised a number of sick people in their homes with the approval of the Ministry of Health, and later also supplied some of the produce to several other patients. As the field grew and developed, the Ministry of Health decided to take action and regulate the industry.
Old set - only NIS 370
With the regulation of the industry, a uniform price was set, which all medical cannabis patients would pay. When the question came up of how much medical cannabis cost, the price was set at a random rate, by the special formula of "Double Live." HI is known as 18 in Gematria. Double 2 is 36 - and from there the price is set at NIS 360 a month. After about a year, the office decided to update the price of medical cannabis and raise it to NIS 370. It doesn't matter if the patient receives 20 grams of cannabis a month, or 200 grams of cannabis a month - the cost, NIS 370, remains the same.
New series - up to NIS 2,000 or more
Following pharmacy reform and the move to a free market format, medical cannabis prices jumped to new highs. Pricing is determined for each product individually, for example, a 10-gram bag costs between NIS 180 and NIS 390 today. Each company competes with its products as it sees fit. Cannabis companies oppose this model, as they say it will hurt them financially.
So how much does medical cannabis cost today?
After implementation of the new regulation, prices jumped to record prices, in the framework of "free market", up to NIS 2,000 and even more. The price is determined by the amount of cannabis the patient receives. , Or a vial of medical cannabis oil. Most companies charge between NIS 180 and 250 for a medical cannabis bag at a pharmacy. Some of the products of other companies come up to NIS 390 per bag.
Full Price List
| Company Name | Product | Name | Super-Pharm | Green-Pharm | Sharm-Pharm | Average |
| BOL Pharma | Oil | BOL T0/C24 CELIXIR24 | $52.48 | $52.48 | $52.48 | $52.48 |
| BOL Pharma | Flower | BOL T0/C24 Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T0/C24 G.Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Oil | BOL T1/C20 CELIXIR20 | $52.48 | $52.48 | $52.48 | $52.48 |
| BOL Pharma | Flower | BOL T1/C20 Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T1/C20 G.Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Oil | BOL T10/C10 EQUATIR10 | $52.48 | -- | $52.48 | $52.48 |
| BOL Pharma | Flower | BOL T10/C10 Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T10/C10 G.Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower | BOL T10/C2 Indica Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T10/C2 Indica G.Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower | BOL T10/C2 Sativa Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T10/C2 Sativa G.Flowers | $55.39 | $42.57 | $55.39 | $51.02 |
| BOL Pharma | Oil | BOL T10/C2 TELIXIR10 | $52.48 | $52.48 | -- | $52.48 |
| BOL Pharma | Oil | BOL T10/C2 TELIXIR10 Indica | $52.48 | $52.48 | $52.48 | $52.48 |
| BOL Pharma | Oil | BOL T10/C2 TELIXIR10 Sativa | $52.48 | $52.48 | $52.48 | $52.48 |
| BOL Pharma | Flower | BOL T15/C3 Indica Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T15/C3 Indica G.Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower | BOL T15/C3 Sativa Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T15/C3 Sativa G.Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Oil | BOL T15/C3 TELIXIR15 | $52.48 | $52.48 | $52.48 | $52.48 |
| BOL Pharma | Flower | BOL T20/C4 Indica Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T20/C4 Indica G.Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower | BOL T20/C4 Sativa Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T20/C4 Sativa G.Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Oil | BOL T20/C4 TELIXIR20 | $52.48 | $52.48 | $52.48 | $52.48 |
| BOL Pharma | Oil | BOL T3/C15 CELIXIR15 | $52.48 | $52.48 | -- | $52.48 |
| BOL Pharma | Flower | BOL T3/C15 Flowers | $55.39 | $42.57 | $55.39 | $51.02 |
| BOL Pharma | Flower Shredded | BOL T3/C15 G.Flowers | $55.39 | $55.39 | -- | $55.39 |
| BOL Pharma | Oil | BOL T5/C10 CELIXIR10 | $52.48 | $52.48 | -- | $52.48 |
| BOL Pharma | Flower | BOL T5/C10 Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T5/C10 G.Flowers | $55.39 | $55.39 | $55.39 | $55.39 |
| BOL Pharma | Oil | BOL T5/C5 EQUATIR5 | $52.48 | $52.48 | -- | $52.48 |
| BOL Pharma | Flower | BOL T5/C5 Flowers | $55.39 | -- | $55.39 | $55.39 |
| BOL Pharma | Flower Shredded | BOL T5/C5 G.Flowers | $55.39 | -- | -- | $55.39 |
| BOL Pharma | Oil | CONNEXIR T1/C20 | $52.48 | -- | -- | $52.48 |
| IMC | Flower | IMC Flow T10C10 PARIS | $63.85 | -- | -- | -- |
| IMC | Flower | IMC Flow T15C3ind LONDON | $63.85 | -- | -- | -- |
| IMC | Flower | IMC Flow T15C3sat DQ | $63.85 | -- | -- | -- |
| IMC | Flower | IMC Flow T20C4ind ROMA | $78.43 | -- | -- | -- |
| IMC | Flower | IMC Flow T20C4sat TLV | $63.85 | -- | -- | -- |
| IMC | Oil | IMC Oil T15C3 CAN | $63.85 | -- | -- | -- |
| Syqe Medical | Evaporation Cartridge | Syqe Inhaler cartridge 60 | $215.74 | -- | -- | $215.74 |
| Bazelet | Flower Shredded | BAZELET T1/C20 | $56.85 | -- | -- | $56.85 |
| Bazelet | Rolled | BAZELET T1/C20 | $56.85 | -- | -- | $56.85 |
| Bazelet | Oil | BAZELET T1/C20 | $62.68 | -- | -- | $62.68 |
| Bazelet | Flower Shredded | BAZELET T10/C10 | $56.85 | -- | -- | $56.85 |
| Bazelet | Rolled | BAZELET T10/C10 | $56.85 | -- | -- | $56.85 |
| Bazelet | Oil | BAZELET T10/C10 | $62.68 | -- | -- | $62.68 |
| Bazelet | Flower Shredded | BAZELET T10/C2 | $56.85 | -- | -- | $56.85 |
| Bazelet | Rolled | BAZELET T10/C2 | $56.85 | -- | -- | $56.85 |
| Bazelet | Oil | BAZELET T10/C2 | $62.68 | -- | -- | $62.68 |
| Bazelet | Flower Shredded | BAZELET T15/C3 | $56.85 | -- | -- | $56.85 |
| Bazelet | Rolled | BAZELET T15/C3 | $56.85 | -- | -- | $56.85 |
| Bazelet | Oil | BAZELET T15/C3 | $62.68 | -- | -- | $62.68 |
| Bazelet | Rolled | BAZELET T20/C4 | $56.85 | -- | -- | $56.85 |
| Bazelet | Oil | BAZELET T20/C4 | $62.68 | -- | -- | $62.68 |
| Bazelet | Flower Shredded | BAZELET T3/C15 | $56.85 | -- | -- | $56.85 |
| Bazelet | Rolled | BAZELET T3/C15 | $56.85 | -- | -- | $56.85 |
| Bazelet | Oil | BAZELET T3/C15 | $62.68 | -- | -- | $62.68 |
| Bazelet | Flower Shredded | BAZELET T5/C10 | $56.85 | -- | -- | $56.85 |
| Bazelet | Rolled | BAZELET T5/C10 | $56.85 | -- | -- | $56.85 |
| Bazelet | Oil | BAZELET T5/C10 | $62.68 | -- | -- | $62.68 |
| Bazelet | Flower Shredded | BAZELET T5/C5 | $56.85 | -- | -- | $56.85 |
| Bazelet | Rolled | BAZELET T5/C5 | $56.85 | -- | -- | $56.85 |
| Bazelet | Oil | BAZELET T5/C5 | $62.68 | -- | -- | $62.68 |
| Better | Flower | BETTER INFLOR T10/C10 IMM | $75.80 | |||
| Better | Flower | BETTER INFLOR T10/C2 SATI | $46.65 | |||
| Better | Flower | BETTER INFLOR T15/C3 ABLE | $75.80 | |||
| Better | Flower | BETTER INFLOR T15/C3 ACTI | $104.96 | |||
| Better | Flower | BETTER INFLOR T15/C3 BALA | $75.80 | |||
| Better | Flower | BETTER INFLOR T15/C3 COMF | $75.80 | |||
| Better | Flower | BETTER INFLOR T15/C3 IND | $104.96 | |||
| Better | Flower | BETTER INFLOR T20/C4 RELI | $104.96 | |||
| Better | Flower | BETTER INFLOR T20/C4 STEA | $84.55 | |||
| Better | Flower | BETTER INFLOR T20/C4 SUPP | $113.70 | |||
| Better | Flower | BETTER INFLOR T3/C15 CURE | $75.80 | |||
| Better | Flower | BETTER INFLOR T5/C10 EFFE | $75.80 | |||
| Better | Oil | BETTER OIL T1/C20 CURE | $75.80 | |||
| Better | Oil | BETTER OIL T1/C20 EFFECTI | $75.80 | |||
| Better | Oil | BETTER OIL T10/C10 IMMUNE | $75.80 | |||
| Better | Oil | BETTER OIL T10/C2 BUILD | $46.65 | |||
| Better | Oil | BETTER OIL T15/C3 COMFORT | $46.65 | |||
| Better | Oil | BETTER OIL T20/C4 SUPPORT | $84.55 | |||
| Teva Adir | Flower | TEVADIR DQ T15/C3 SAT.FLO | $72.89 | $72.89 | ||
| Teva Adir | Flower | TEVADIR FEDE T15/C3 SAT F | $72.89 | |||
| Teva Adir | Flower | TEVADIR JUAN T5/C10 FLOWE | $72.89 | |||
| Teva Adir | Flower | TEVADIR ML T15/C3 IND.FLO | $72.89 | $72.89 | ||
| Teva Adir | Flower | TEVADIR MOR C15/T3 FLOWER | $78.72 | |||
| Teva Adir | Oil | TEVADIR MOR T3/C15 OIL | $72.89 | |||
| Teva Adir | Flower | TEVADIR RM T10/C10 FLOWER | $78.72 | |||
| Teva Adir | Oil | TEVADIR T10/C10 OIL | $78.72 | |||
| Teva Adir | Oil | TEVADIR T10/C2 OIL | $72.89 | |||
| Teva Adir | Oil | TEVADIR T15/C3 OIL | $72.89 | |||
| CannDoc | Flower | T1/C20 CBD CANNDOC | $56.85 | |||
| CannDoc | Flower | T10/C2 INDICA CANNDOC | $52.48 | |||
| CannDoc | Flower | T10/C2 SATIVA CANNDOC | $52.48 | |||
| CannDoc | Flower | T15/C3 INDICA CANNDOC | $56.85 | |||
| CannDoc | Flower | T15/C3 SATIVA CANNDOC | $56.85 | |||
| CannDoc | Flower | T20/C4 INDICA CANNDOC | $58.31 | |||
| CannDoc | Flower | T20/C4 SATIVA CANNDOC | $58.31 | |||
| CannDoc | Flower | T3/C15 CBD CANNDOC | $49.56 | |||
| CannDoc | Flower | T5/C10 CBD CANNDOC | $49.56 | |||
| CannDoc | Flower | T5/C5 CANNDOC INFLOR | $54.23 | |||
| CannDoc | Oil | T5/C5 CANNDOC OIL | $55.39 | |||
| Rafa | Oil | AXIBAN SL DROPS T1/C20 | $76.97 | $78.72 | $76.97 | $77.55 |
| Rafa | Oil | AXIBAN SL DROPS T10/C10 | $55.10 | $58.31 | $55.10 | $56.27 |
| Rafa | Oil | AXIBAN SL DROPS T10/C2 | $44.02 | $46.65 | $44.02 | $44.90 |
| Rafa | Oil | AXIBAN SL DROPS T15/C3 | $65.89 | $67.06 | $65.89 | $66.18 |
| Rafa | Oil | AXIBAN SL DROPS T3/C15 | $65.89 | $67.06 | $65.89 | $66.18 |
| Rafa | Oil | AXIBAN SL DROPS T5/C10 | $55.10 | $58.31 | $55.10 | $56.27 |
| Rafa | Oil | AXIBAN SL DROPS T5/C5 | $50.44 | $51.02 | -- | $50.73 |
| Seach | Flower | Canary Indica T10/C2 | $52.48 | $52.48 | -- | $52.48 |
| Seach | Flower | Canary Indica T15/C3 | $53.94 | $46.65 | -- | $50.44 |
| Seach | Flower | Canary Indica T20/C4 | $53.94 | $46.65 | -- | $50.44 |
| Seach | Flower | Canary Sativa T10/C2 | $52.48 | $49.56 | -- | $51.02 |
| Seach | Flower | Canary Sativa T15/C3 | $53.94 | $52.48 | -- | $53.35 |
| Seach | Flower | Canary Sativa T20/C4 | $53.94 | $46.65 | -- | $50.44 |
| Seach | Oil | Canary T0/C24 | $61.22 | $46.65 | -- | $53.94 |
| Seach | Flower | Canary T0/C24 | $52.48 | $46.65 | -- | $49.56 |
| Seach | Oil | Canary T1/C20 | $61.22 | $46.65 | -- | $53.94 |
| Seach | Flower | Canary T1/C20 | $52.48 | $46.65 | -- | $49.56 |
| Seach | Oil | Canary T10/C10 | $55.39 | $46.65 | -- | $51.02 |
| Seach | Flower | Canary T10/C10 | $52.48 | $46.65 | -- | $49.56 |
| Seach | Oil | Canary T10/C2 | $49.56 | $46.65 | -- | $48.10 |
| Seach | Oil | Canary T15/C3 | $58.31 | $46.65 | -- | $52.48 |
| Seach | Oil | Canary T20/C4 | $61.22 | $46.65 | -- | $53.94 |
| Seach | Oil | Canary T3/C15 | $58.31 | $46.65 | -- | $52.48 |
| Seach | Flower | Canary T3/C15 | $52.48 | $46.65 | -- | $49.56 |
| Seach | Oil | Canary T5/C10 | $55.39 | $46.65 | -- | $51.02 |
| Seach | Flower | Canary T5/C10 | $49.56 | $53.94 | -- | $51.90 |
| Seach | Flower | Nitzan T0/C24 | $58.31 | $58.31 | -- | $58.31 |
| Seach | Oil | Nitzan T0/C24 | $72.89 | -- | -- | $72.89 |
| Seach | Flower | Nitzan T1/C20 | $58.31 | $58.31 | -- | $58.31 |
| Seach | Oil | Nitzan T1/C20 | $72.89 | -- | -- | $72.89 |
| Seach | Oil | Nitzan T10/C10 | $58.31 | $58.31 | -- | $58.31 |
| Seach | Flower | Nitzan T10/C10 | $52.48 | $52.48 | -- | $52.48 |
| Seach | Oil | Nitzan T10/C2 | $49.56 | $51.02 | -- | $50.44 |
| Seach | Flower | Nitzan T10/C2 Indica | $60.93 | $60.93 | -- | $60.93 |
| Seach | Flower | Nitzan T10/C2 Sativa | $60.93 | $60.93 | -- | $60.93 |
| Seach | Oil | Nitzan T15/C3 | $56.85 | $56.85 | -- | $56.85 |
| Seach | Flower | Nitzan T15/C3 Indica | $68.22 | $68.22 | -- | $68.22 |
| Seach | Flower | Nitzan T15/C3 Sativa | $68.22 | $68.22 | -- | $68.22 |
| Seach | Oil | Nitzan T20/C4 | $61.22 | -- | -- | $61.22 |
| Seach | Flower | Nitzan T20/C4 Indica | $72.59 | $72.59 | -- | $72.59 |
| Seach | Flower | Nitzan T20/C4 Sativa | $72.59 | $72.59 | -- | $72.59 |
| Seach | Flower | Nitzan T3/C15 | $55.39 | -- | -- | $55.39 |
| Seach | Oil | Nitzan T3/C15 | $64.14 | -- | -- | $64.14 |
| Seach | Oil | Nitzan T5/C10 | $58.02 | $58.02 | -- | $58.02 |
| Seach | Flower | Nitzan T5/C10 | $52.48 | $52.48 | -- | $52.48 |
| Seach | Flower | Nitzan T5/C5 | $52.48 | $52.48 | -- | $52.48 |
| Seach | Oil | Nitzan T5/C5 | $58.02 | -- | -- | $58.02 |
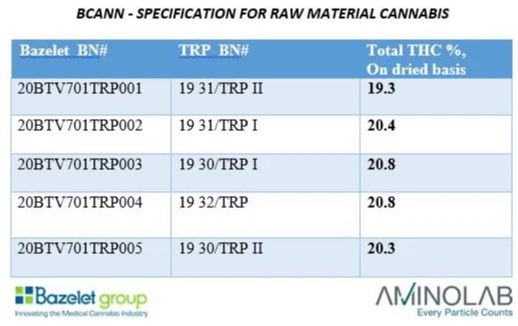
Next week in pharmacies: "Bazelet" company with first import of overseas cannabis from the T20 category. "Ensure continuous supply of high-quality, clean cannabis," says the company's CEO. Accurate THC percentages will be displayed for the first time.
Bazelet first imported about half a ton of cannabis from Portugal last week to fill the shortage of high-quality cannabis pharmacies from the T20 category. Early next week, the new products should be available in patient pharmacies.

Bazelet's imported cannabis comes from EU-GMP standard incubators and is the first in Israel to show an accurate percentage of THC and not just the category (T20 in this case), so patients can know better what they are getting - in this case between 19.3% and 20.8% THC.
Although the Ministry of Health has promised that there will be an obligation to present an accurate quantity of active ingredients as of 1.1.20, the rest of the manufacturers are still not properly marking and the products may contain between 16% and 24% of THC in the case of T20 and a similar gap in other categories.

Cannabis, weighing 400 kilograms, was shipped to Israel by the recently acquired cannabis Portuguese company Terra Verde, acquired by the European cannabis company EMMAC (EMMAC).
Cannabis will be marketed in Israel under the Bazelet home brand, "BCANN" (BCANN), underneath which cannabis products purchased from independent growing companies in Israel and worldwide. At the same time, “Bazelet” manufactures branded products for other companies.
The new cannabis imported by the “Bazelet” company from Portugal Packed in the company plants The BCANN cannabis flower T20 / C4 of the BCANN series of Bazelet cannabis flower T20 of the BCANN series from Portugal
Meir Ariel, CEO of the Bazelet Group, said: "As a leading player in the medical cannabis industry in Israel, the Bazelet Group does everything it can to ensure a continuous supply of premium, high-quality and clean medical cannabis."
Ariel also addressed the issue of export, saying: “Israeli patients are our top priority and we will take care of the first. We will not export cannabis abroad until we resolve their plight and ensure the continuity of their treatment. ”
One of the reasons for C20's cannabis shortage, the one imported by Bazelet, is that in the past, companies released unreliable data and now, after being determined to comply with the standards, find that the same products contained less than declared - the Ministry of Health explains.
The shipment that Bazelet brought is the second to arrive in Israel from Portugal after a few years before it did so, "CannDoc", which imported 250 kg from the facilities of "Tlray". There, too late, hope to transfer Portuguese cannabis to the pharmacies soon.
Thus, a total of 650 kg of medical cannabis has been imported to Israel so far - all from Portugal, which is the largest medical cannabis exporter in Europe. Most Canadian companies, as well as Israeli companies, set up facilities in Portugal to export cannabis throughout Europe.
Source: Cannabis Magazine Israel

Kyle Jaeger
Comprehensive marijuana reform will be at the center of another congressional hearing next week, a key committee announced on Wednesday.
Two months after the House Judiciary Committee approved the Marijuana Opportunity, Reinvestment and Expungement (MORE) Act, a comprehensive cannabis legalization bill sponsored by Chairman Jerrold Nadler (D-NY), the issue will be the subject of another discussion in an Energy and Commerce Subcommittee.
The topic will be taken up by the panel’s Subcommittee on Health on January 15.
“As public opinion continues to evolve and cannabis policies change at all levels of government, it’s important to bring federal agency officials together to discuss current and future federal cannabis policies,” Energy and Commerce Chairman Frank Pallone (D-NJ) and Health Subcommittee Chairwoman Anna Eshoo (D-CA) said in a joint statement. “We’re particularly interested in examining the implications of changing marijuana’s schedule listing, the potential of cannabis research, and federal efforts to review and approve cannabidiol products.”
NEWS: Health Subcommittee announces a legislative hearing on current and future federal cannabis policies.
It’s not clear if the hearing, titled the “Cannabis Policies for the New Decade,” will focus on specific legislation such as the MORE Act. So far, the committee has not listed any witnesses who will testify at the meeting.
“The Energy and Commerce committee taking up marijuana reform is an unprecedented and welcome development,” Justin Strekal, political director of NORML, told Marijuana Moment. “As the MORE Act continues to move through the process, the House is poised to become the first chamber of Congress in history to pass a bill to end prohibition.”
Nadler previously told Marijuana Moment that his office was “carrying on conversations” with other committees that his bill had been referred to, such as Energy and Commerce, to see if they’d waive jurisdiction in order to advance it more quickly to the full floor.
“I don’t anticipate that to be a big problem,” he said. “We are looking forward to moving this to the floor at an appropriate time when we’ve done some more educational work and have the votes.”
The Small Business Committee said this week that it’s agreed to yield on the bill.
Marijuana Moment has reached out to staffers on each of the panels, but while several responded, they said plans haven’t been finalized yet. A National Resources Committee communications official said he “wouldn’t put us down as a potential obstacle” on the legislation’s path to the floor.
The legislation has also been referred to Agriculture, Education and Labor, Ways and Means, and Oversight and Reform.
Nadler’s MORE Act has been a subject of intense interest for cannabis reform advocates, who view it as an ideal vehicle to end federal prohibition and repair the harms of the drug war, particularly for communities of color.
Beside descheduling marijuana, the bill would expunge the records of those with prior cannabis convictions and impose a five percent tax on sales, revenue from which would be reinvested in those disproportionately impacted communities.
It would also create a pathway for resentencing for those incarcerated over marijuana offenses, and it would provide protections ensuring that immigrants couldn’t be denied citizenship over cannabis. Additionally, the legislation would prevent federal agencies from denying public benefits or security clearance due to marijuana use.
It’s not clear if and when Energy and Commerce plans to hold a possible markup to vote on the legislation following Wednesday’s subcommittee hearing but, given the number of other panels that could also choose to take it up and the pressure from advocates to advance the legislation this Congress, it seems likely that such action will be scheduled in a timely fashion.
Source: Marijuana Moment